Find out why seed oils make sunburned skin more inflamed and how avoiding them protects your collagen and keeps you looking young

PUFA-Project: Scientific References on Seed Oil Toxicity
Table of Contents
- Mission:
- Basic Principles
- The Excessive Seed Oil Hypothesis
- Historically adipose tissue contained a fraction of the PUFA found in adipose tissue today.
- How Seed Oils Lead to Obesity and Diabetes:
- Adipose Fatty Acid Profile Changes from High PUFA Intake
- Adipose Toxicity a.k.a. “sick fat”
- More PUFA in Adipose Correlates with Higher Rates of Heart Attack
- ARDS (Acute Respiratory Distress Syndrome)
- Atherosclerosis
- Asthma
- “Linoleic acid metabolite drives severe asthma by causing airway epithelial injury”
- “Lipid mediator profiles differ between lung compartments in asthmatic and healthy humans”
- Blood clots
- Brain development abnormalities
- Cancer
- Cancer Types
- Crohn’s Disease
- Diabetes (See Insulin Resistance and Diabetes)
- Endothelial Dysfunction
- Fatty Liver
- Gut Inflammation and Colitis
- Gut Microbiome Abnormalities
- Heart Attack (see Atherosclerosis)
- Heart Failure
- HDL Dysfunction and lowered HDL
- Insulin Resistance and Diabetes
- Macular Degeneration
- Mitochondrial Dysfunction
- Muscle Atrophy
- Obesity
- Omega-3 Fatty Acid Depletion
- Oxidized Cholesterol (See Atherosclerosis, PUFA Promote Oxidized LDL and HDL)
- Pregnancy Complications
- Schizophrenia
- Stomach Acid Deficit
- Sudden Cardiac Death
- Sunburn
- Cognitive Dysfunction
- Loss of Self Control (IR induces hypoglycemia symptoms)
- MECHANISMS of TOXICITY: Mitochondrial Dysfunction and Cytotoxicity
- PUFA in “High Fat” Diets
- PUFA IN LARD
- Vitamin E in oxidized Seed oils has Pro-Oxidant Activity
- Points of confusion
- How PUFA enter/ build brain
- Seed oil Consumption data sources
- Prediabetes and diabetes trend data sources
Mission:
- To promote consumer awareness of the evidence that PUFA overconsumption from seed oils that are wrongly promoted as healthy may be the underlying driver of most non-infectious diseases
- To increase health professionals’ awareness of the role of high-PUFA refined oils (The Hateful Eight) in driving non-infectious disease
- To support businesses that avoid seed oils and offer consumers healthy fats and oils (click here for products)
- To support global human nutrition and health by elevating culinary skills that wean us off seed oils
Basic Principles
PUFA stands for polyunsaturated fatty acid. PUFAs react with oxygen and deteriorate into toxins. Saturated fats do not. When we eat too much PUFA, it overwhelms our metabolism with something called “oxidative stress.” Oxidative stress disrupts homeostasis, is the underlying process behind our obesity and chronic disease epidemics, and ultimately ages and kills us.
We’re all going to die from oxidative stress. But seed oils accelerate that process. They make us chronically il many years before we die, and they bring about an early death. Whole foods that contain PUFA also contain antioxidants, so PUFA-rich foods (including the seeds from which these oils are derived) are not necessarily a problem. The problem is the refined oils derived from these seeds.
Today, Americans consume unhealthy amounts of seed oils, primarily because Harvard and the American Heart Association (AHA) actively promote them. Supposedly, these oils are healthy due to their high PUFA content and cholesterol-lowering ability.
Unfortunately, healthcare professionals rarely question the veracity of the AHA and Harvard. We do not learn the history of the Harvard-AHA alliance and of the AHA’s dependence on funding from companies selling products made with seed oil. Most healthcare professionals are also unaware of the scientific fraud perpetrated by the men who originally proposed the idea that seed oils are healthy and saturated fats are unhealthy.
This article is continued below...(scroll down)
It is our position that current levels of seed oil consumption are the main driver of the obesity and chronic disease epidemics that now represent an existential threat to human populations around the world.
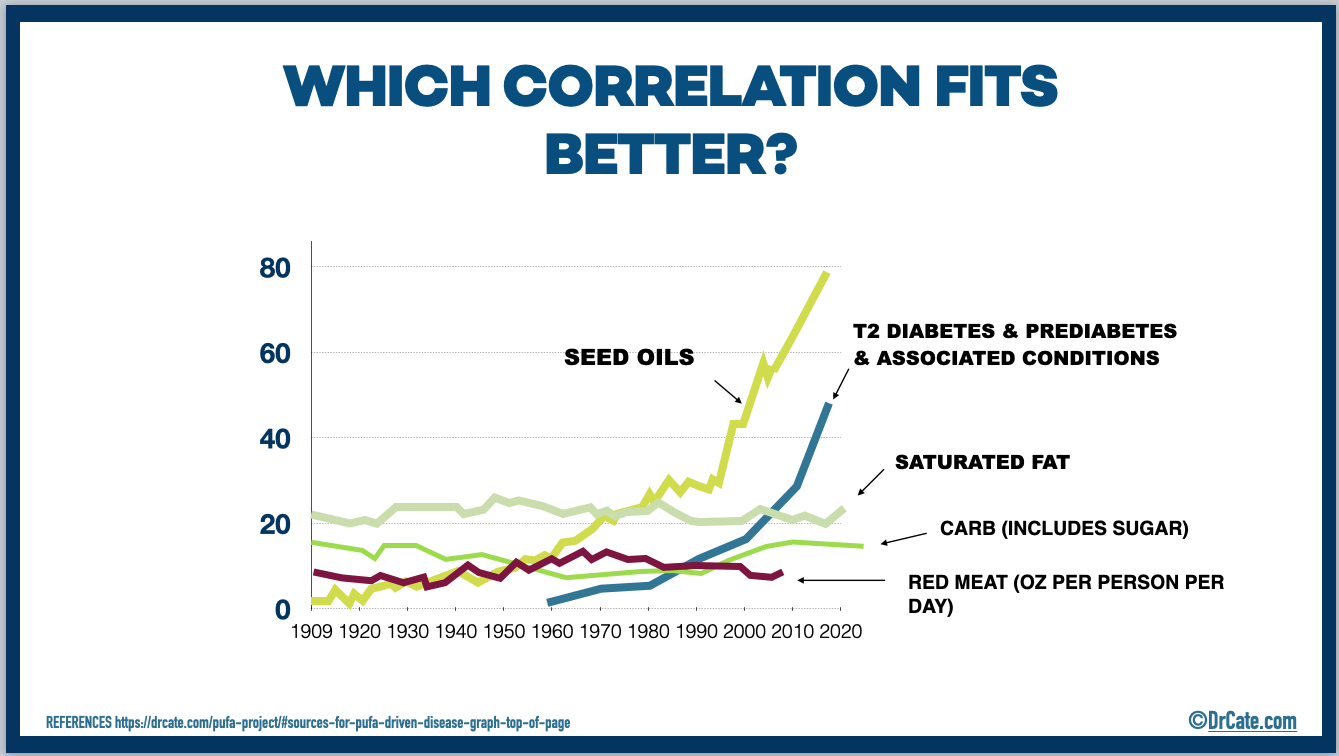
The Excessive Seed Oil Hypothesis
Today’s historically unprecedented consumption of seed oil is the obvious outlier dietary change that’s taken place over the past 70 years (see graph, above), and is, therefore, the best, most logical candidate for the primary driving factor behind metabolic and inflammatory diseases.
To learn more about how eliminating seed oils and optimizing your nutrition can help you recover from many chronic conditions, please visit this page.
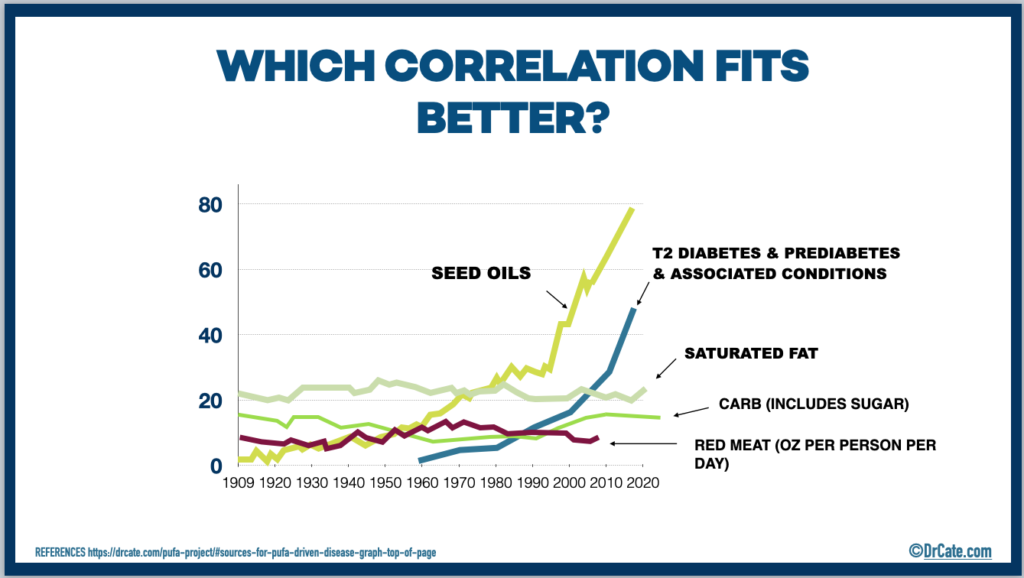
Historically adipose tissue contained a fraction of the PUFA found in adipose tissue today.
The below reference shows the time period 1950-2000. When considering 1909-2020, the differences are more dramatic.
“Increase in Adipose Tissue Linoleic Acid of US Adults in the Last Half-Century” Authors: Stephan J Guyenet and Susan E Carlson (image below)
Non-infectious chronic diseases all share a common root cause: oxidative stress. Seed oils are the primary driver of oxidative stress when consumed in excess. The chemical principle is that seed oils are high in easily oxidizable polyunsaturated fats (PUFA) and will endure uncontrolled, non-enzymatic, spontaneous oxidative attacks while in our cell membranes, lipoproteins, organelle membranes, serum, interstitial spaces and so on, all of which will promote localized oxidative stress and localized disruption of homeostasis. The body can handle some of these spontaneous reactions with our endogenous antioxidant enzymes (i.e. catalase) vitamins (i.e. tocopherol) and other compounds (i.e. glutathione). However when our total body burden of PUFA exceeds the capacity of these systems, the oxidative stress disrupts homeostasis and, if sustained ,can lead to myriad disease states.
Reference supporting the notion that excessive PUFA intake causes oxidative stress
- Title: A high linoleic acid diet increases oxidative stress in vivo and affects nitric oxide metabolism in humans LINK: https://pubmed.ncbi.nlm.nih.gov/9844997/
- Title: Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats LINK: https://www.sciencedirect.com/science/article/abs/pii/S0891584915000891
How Seed Oils Lead to Obesity and Diabetes:
One of the metabolic adaptations available to cells is to use an alternative fuel, specifically sugar. Unfortunately, cells can utilize more sugar than the bloodstream can carry, causing hypoglycemia symptoms. These symptoms, in turn lead to poor food choices.
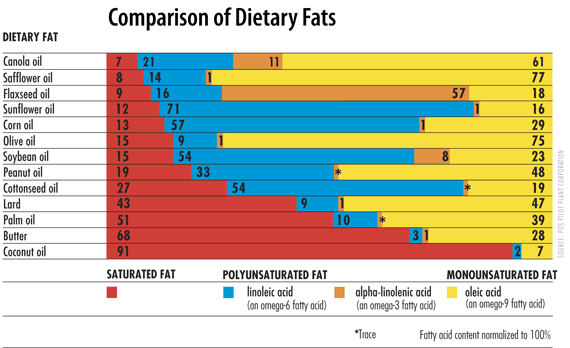
Saturated fat resists oxidation in the body and during cooking. Traditional cooking fats and oils are relatively higher in saturated fat (see butter and lard) and lower in PUFA.
This page offers references supporting that the many metabolic impairments that may be making you sick all stem in part or wholly from excessive seed oil consumption. (Also described in my books.)
Our claim is that if all Americans stopped seed oil consumption today and started cooking and buying products made with only traditionally produced fats and oils, in 3 months we would all be noticeably healthier and in 3 years our healthcare costs could plummet by 90% or more.
Seed Oils Make You Crave Carbs, Sugar
Because PUFA can increase the body’s requirements for sugar, people’s appetites are drive towards refined flours and sugar and thus their ability to cut down on these sources of excess calories is impaired. Therefore, the most logical first step for professionals giving dietary advice is to recommend the substitution of seed oils for traditional fats and oils. When consumed at breakfast (or the first meal of the day) this advice will make it easier for people to reduce calories from refined flour and sweets starting on day one and should be emphasized at the outset of all nutrition-optimization programs.
How Genetics Plays a Role: The Personal PUFA Threshold Concept.
PUFA first concentrates in the liver, omentum, and/or body fat (adipose). The location is likely genetically determined. Over time, body fat PUFA concentration will reflect habitual PUFA intake. The high concentration of PUFA throughout the body will ultimately cause a different set of diseases in different individuals, and this is also likely genetically determined. For example, some people develop atherosclerosis before they develop diabetes, and vice versa. Some people develop autoimmune disease, while others develop cancer.
The Excessive Seed Oil Hypothesis explains these differences as follows: the body burden of PUFA at which a given tissue’s antioxidant defense system is overwhelmed is the determining factor influencing what diseases an individual will develop.
Tasks
- Continue to compile research connecting PUFA consumption to multiple health conditions. (i.e. fatty liver, dysbiosis, etc.)
- Create videos that explain in simple terms how PUFA consumption promotes multiple health conditions
- Evaluate the potential of RBD-oil free certification for manufacturers/resellers
- Identify partners w/ RBD-free products
Who is Warning Us That Excess PUFA is Toxic?
(just gettin’ started on this, more details to come)
-
Eric Decker, PhD
-
Martin Grootveld, PhD
-
Gerhardt Spiteller, PhD
-
Glen D Lawrence Department of Chemistry and Biochemistry, Long Island University, Brooklyn, NY
-
- “PUFAs are important for production of a wide range of bioactive substances in the body, but like some vitamins and essential minerals, may become toxic when consumed in excess.
- PUFAs can be oxidized in a spontaneous chemical oxidation process that does not require enzymes and would not be regulated in cells by normal processes.”
- “…all-cause death rate is higher in humans with low compared with normal or moderately elevated serum total cholesterol”
- “numerous adverse effects [emerge from] increasing dietary PUFAs or carbohydrate relative to SFAs”
The Body Burns PUFA in Attempt to Detoxify Itself, but That Can Backfire
“Mammals preferentially oxidize PUFAs via the beta-oxidation pathway in mitochondria for ATP production, or in peroxisomes to recycle excess PUFAs into SFAs and MUFAs to eliminate a portion of these reactive nutrients and prevent formation of potential toxins, particularly in the fetus and infant” [source]
Alphabetical List of Health Problems Associated with Excessive High-PUFA Seed Oil Consumption (See also pale blue navigation bar above)
Adipose Fatty Acid Profile Changes from High PUFA Intake
Article Title: “Adipose tissue biomarkers of fatty acid intake”
Key findings:
- Dietary PUFA increases adipose sat fat and decreases adipose oleic acid
- DHA plateaus such that supplementing DHA beyond that found in 100gm fish per week fails to increase adipose concentration. The authors do not speculate that the omega-3 may spontaneously oxidize beyond this amount, but I suspect that may be a contributing factor
ARticle link: https://academic.oup.com/ajcn/article/76/4/750/4677442
- Article Quotes:
- Adipose tissue oleic acid was correlated inversely with vegetable fatty acid and PUFAs (Table 3) but correlated directly with animal fatty acid and SFAs; it showed no correlation with its corresponding dietary fatty acid.
- 30% of the variability in total adipose tissue trans fatty acids is due to soybean oil, which is used in the everyday cooking of rice, beans, and fried food.
- Margarine and baked products were the next major source of trans fatty acids.
- In contrast, the partially hydrogenated soybean oil used for cooking does not contribute to trans fatty acid intake in other population [Costa Rica]
- It is remarkable that adipose tissue oleic acid shows the best correlation with saturated fat intake, even better than that of any adipose tissue SFA. Positive correlations between serum MUFAs and dietary SFAs have been reported by others
- We did not find a correlation between dietary PUFAs and adipose tissue arachidonic acid, which suggests that there is little endogenous conversion of linoleic acid to arachidonic acid.
- Adipose tissue trans fatty acids also showed moderate correlations with dietary n?6 and n?3 PUFAs because the main source common to them all is soybean oil.
- This relation appeared to reach a plateau at an intake of ?100 g/wk. We cannot determine whether this association reflects a real biological phenomenon or a combination of measurement error and a narrow range of intake, particularly in a population whose intake of fish is low.
Adipose Toxicity a.k.a. “sick fat”
PUFAs Concentrate in Our Body Fat over Time
More PUFA in Adipose Correlates with Higher Rates of Heart Attack
Article “Can linoleic acid contribute to coronary artery disease?”
Source: https://pubmed.ncbi.nlm.nih.gov/8192728/
“Lipid peroxidation of polyunsaturated fatty acids in normal and obese adipose tissues.”
- Summary:
- Not all obesity is alike. Some folks have body fat that is distressed by inflammation and behaves like it’s chronically infected. Termed “adiposopathy” and sometimes called “sick fat” people with this condition have body fat that does not secrete leptin to promote satiety and instead secretes inflammatory cytokines, which puts them at higher risk of heart attack, stroke, blood clots, and COVID complications.
- Article Quotes
- Adipose tissue inflammation has also been linked to enhanced metabolism of polyunsaturated fatty acids (PUFAs)
- The non-enzymatic peroxidation of PUFAs and of their 12/15-lipoxygenase-derived hydroperoxy metabolites leads to the generation of the reactive aldehyde species 4-hydroxyalkenals.
- This review shows that 4-hydroxyalkenals, in particular 4-hydroxynonenal, play a key role in lipid storage homeostasis in normal adipocytes. Nonetheless, in the obese adipose tissue an increased production of 4-hydroxyalkenals contributes to the inflamed phenotype.
“Increased Adipose Protein Carbonylation in Human Obesity”
- The article shows that abnormal adipose function stems from oxidative stress.
- Article quotes:
- The level of protein carbonylation was directly correlated with adiposity and serum free fatty acids (FFAs). These results suggest that in human obesity oxidative stress is linked to protein carbonylation and such events may contribute to the development of insulin resistance.
- production of ROS by mitochondria inhibits the proliferation of preadipocytes without inducing apoptosis
ARDS (Acute Respiratory Distress Syndrome)
“Why respiratory infections are more deadly in diabetics”
They have an exaggerated inflammatory response. MERS-related mouse study published in october 2019.
Article quote
- …The exaggerated inflammatory response appears related to higher PUFA (mostly Linoleic acid) levels… (see next ref)
“An Increase in Serum C18 Unsaturated Free Fatty Acids as a Predictor of the Development of Acute Respiratory Distress Syndrome”
https://pubmed.ncbi.nlm.nih.gov/8674324/
Article quote
- the ratio of C18 unsaturated fatty acids linoleate and oleate to fully saturated palmitate, C16:0) increased and predicted the development of ARDS in at-risk patients. Serum samples from the 30 patients, obtained within 24 hrs from the onset of sepsis, trauma, or development of ARDS
“Neutrophil-derived epoxide, 9,10-epoxy-12-octadecenoate, induces pulmonary edema.”
https://pubmed.ncbi.nlm.nih.gov/3148792/
Article quotes
- These results indicate that leukotoxin biosynthesized by neutrophils might be closely related to the genesis of inflammatory edema.
- (These authors defined Leukotoxina as a specific product of linoleic acid oxidation, 9,10-epoxy-12-octadecenoate )
Surfactant phospholipids: synthesis and storage.
https://www-ncbi-nlm-nih-gov.prx.hml.org/pubmed/1566854
Article quotes:
- Pulmonary surfactant, a complex consisting of 90% lipids and 10% specific proteins, lines the alveoli of the lung and prevents alveolar collapse and transudation by lowering the surface tension at the air-liquid interface.
- Dipalmitoylphosphatidylcholine constitutes approximately 50% of the surfactant lipids and is primarily responsible for the surface tension-lowering property of the surfactant mixture.
- surfactant phospholipids are produced at the endoplasmic reticulum of the alveolar type II epithelial cells.
- The characteristic lamellar bodies in these cells serve as storage depot for the surfactant before this is secreted onto the alveolar surface
Five Decades with Polyunsaturated Fatty Acids: Chemical Synthesis, Enzymatic Formation, Lipid Peroxidation and Its Biological Effects
https://www.nature.com/articles/pr1986144
Article quotes:
- Adding linoleic acid oxidation products stimulates further oxidation in lung mitochondria and microsomes. In article quote “…Lipid Peroxidation Stimulated by Linoleic Acid Hydroperoxide of Rat Lung Mitochondria and Microsomes”
Surfactant protein d, a marker of lung innate immunity, is positively associated with insulin sensitivity.
https://www-ncbi-nlm-nih-gov.prx.hml.org/pubmed/20086254
Diabetics have lower levels of surfactant protein d than non diabetics, and weight loss in these patients lowered their surfactant protein d further.
This is as you would expect IF 1) PUFAs oxidize surfactant protein d thus reducing levels and 2) Weight loss in DM2 releases more PUFAs and further shifts the FA composition within the type2 alveolar cell towards oxidation.
Altered Lipid Composition of Surfactant and Lung Tissue in Murine Experimental Malaria-Associated Acute Respiratory Distress Syndrome
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4666673/
Article quotes;
- Inflammation and increased endothelial permeability are important features of both human and mouse MA-ALI/ARDS malaria associated lung injury.
- Oxidative degradation of lipids results in the accumulation of reactive aldehydes, such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), which are highly cytotoxic [16, 17].
- An altered lipid profile and increased levels of lipoperoxidation end products have been found in plasma from patients with ARDS of different aetiologies
Alteration of fatty acid profiles in different pulmonary surfactant phospholipids in acute respiratory distress syndrome and severe pneumonia.
https://www.ncbi.nlm.nih.gov/pubmed/11208632/
PUFA of certain surfactants elevated by 3+ x in ARDS v healthy controls, MUFA not much different, other surfactants are elevated in MUFA but as a percentage elevated less than the PUFA elevations (downloaded PDFto 27 inch mac search by title)
Surfactant alteration and replacement in acute respiratory distress syndrome
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC64803/
Role of hypercoagulability touched on in one of the figures
Increased saturated phospholipid in cultured cells grown with linoleic acid
From https://link.springer.com/article/10.1007/BF02796331
Linoleic acid alters phospholipids
Hx of ARDS
Acute Respiratory Distress Syndrome in Combat Casualties: Military Medicine and Advances in Mechanical Ventilation
Military Medicine VOLUME 171 NOVEMBER 2006 NUMBER 11
Science-based blog on the ARDS PUFA connection
https://yelling-stop.blogspot.com/2020/06/does-consumption-of-omega-6-seed-oils.html
Atherosclerosis
What is the cause?
The AHA and most lipidologists (wrongly) claim:
LDL is inherently prone to oxidation regardless of diet, thus “bad.” The AHA largely denies that PUFAs, as oxidation prone fatty acids, can promote LDL oxidation prior to uptake by endothelial cells. Their current thinking is that the PUFAs in LDL can only become oxidized AFTER LDL enters the endothelial wall, not while being cooked, not during digestion, not in the liver, not while in other lipoproteins, and not while in circulation. This goes against most of the available science.
Their hypothesis is that LDL becomes oxidized in the endothelial wall as a byproduct of smoking, insulin resistance, red meat consumption (via TMAO), and multiple other factors. All variables under consideration are also related to oxidation processes and therefore are also driven by PUFA consumption. Because polyunsaturated fatty acids can lower LDL, the AHA and most lipidologists believe actually recommend we consume more PUFA, not less.
The Excessive Seed Oil Hypothesis (correctly) claims:
On a high-seed oil diet, all lipoproteins are prone to oxidation. The antioxidant defense mechanisms may at first prevent oxidized lipoproteins from causing clinically meaningful disease. However, on a high seed oil diet, the oxidative stress ultimately will exceed the capacity for a person’s antioxidant systems to control lipid oxidation. Once a person’s antioxidant capacity has been depleted, continued excessive seed oil consumption turns all lipoproteins into agents of atherosclerosis.
Excessive PUFA oxidizes LDL in stages.
- When LDL is only minimally oxidized, aka “minimally modified” (mmLDL), the apo B is not affected and therefore mmLDL can bind LDL receptors and enter endothelial cells.
- Non LDL lipoproteins are not endocytosed. LDL, however, is.
- Thus mmLDL serves as the lipoprotein delivery vehicle of oxidized cholesterol and PUFA into endothelial cells.
- More fully oxidized LDL (oxLDL) can not bind LDL receptors but can bind scavenger receptors. This has 2 consequences that promote atherosclerosis
-
- The half-life of oxLDL in the vessel lumen exceeds its ability to remain in suspension, thus oxLDL are essentially “stranded” in circulation and ultimately will end up deposited on the lumen of the arterial wall where they can directly damage endothelial cells
- Additionally, the deposition of oxLDL onto endothelial cells in a blood vessel lumen will trigger adhesion molecules that attract white blood cells and lead to further oxidation-induced inflammation, foam cell formation, and endothelial damage.
-
The impact on non-LDL lipoproteins plays out as follows:
- Oxidized non LDL lipoproteins promote atherosclerosis by the following mechanism: Oxidized PUFA in chylomicrons and other triglyceride-rich lipoproteins disrupt the offloading of triglyceride fat, causing free oxidized PUFA and ox cholesterol to “spill” onto the endothelial cell surface.
The role of sugar:
- Sugar accelerates the dysfunction of LDL and other lipoproteins.
- It’s important to note that sugar consumption alone does not promote insulin resistance, and the role sugar plays is indirect and primarily related to the fact that sugar and refined flours displace nutrients.
- The effect of dietary sugar is minimal until blood sugar levels are chronically high, which occurs after a person develops insulin resistance. High blood sugar becomes intensely problematic after a person develops T2DM, not before. Sugar may accelerate the problem as follows
- Glycating apoproteins, impairing their function.
- Glycating receptors for all lipoproteins and impairing:
- Delivery of triglycerides and causing blood triglyceride elevations
- Exchange of apoproteins between HDL and other lipoproteins, reducing HDL levels.
The meaning of “small dense” LDL and other lipoproteins
- The formation of oxidized (aka “small dense”) LDL, HDL, other lipoproteins, and atherosclerosis is not entirely genetic or age-related, but rather a result of how genetics and age influence factors 1-3 above.
References supporting the Excessive Seed-oil Atherosclerosis Hypothesis:
Title: “Vegetable oil or animal fat oil, which is more conducive to cardiovascular health among the elderly in China?”
Article link https://www.sciencedirect.com/science/article/pii/S0146280622003826
Highlights:
- The study included more than 15,000 Chinese people 65 and older
- The article looked at the link between dietary fat and atherosclerotic diseases including strokes and heart conditions.
- The authors concluded, “Cooking with lard/other animal fat oil is more beneficial to cardiovascular health in older Chinese.”
- The difference was pretty clear, with 31.68 % of vegetable oil users having disease compared to 17.46 of animal fat users. You don’t need to be a statistician to see that there’s a big difference.
- Other studies in China also showed “consumption of SFA-rich [saturated fatty acid] animal fatty oils is associated with a reduced risk of cardiovascular disease”
- The vast majority, 86%, of people over age 65 now use cook with one or more of the Hateful 8 vegetable oil rather than traditional lard or other animal fat.
- The authors try to sound the alarm on trusting the AHA: “Dietary guidelines should seriously consider the health effects of substituting vegetable/gingili oil for lard/other animal fat oil for different populations.”
TITLE: “The effect of short-term canola oil ingestion on oxidative stress in the vasculature of stroke-prone spontaneously hypertensive rats”
- This article showed that canola oil reduced the antioxidant capacity of the animal’s blood/tissues, and promoted “lipid peroxidation” in cholesterol carrying particles, which is consistent with what the other articles in this segment say.
- Article link: https://lipidworld.biomedcentral.com/articles/10.1186/1476-511X-10-180
- Source Lipids Health Dis, October 2011, 17;10:180.
Title:
Title: “Omega-6 vegetable oils as a driver of coronary heart disease: the oxidized linoleic acid hypothesis” (Dr. James O’Keefe)
- This article proposes linoleic acid consumption as the main agent of atherosclerosis.
- ARticle link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6196963/
- Article quotes
- “…patients who have heart disease consume more omega-6 linoleic acid than those without heart disease”
- what causes LDL to become oxidised in the first place?
- the oxidation of LDL was initiated by the oxidation of linoleic acid contained within the LDL particles.
- Indeed, linoleic acid is the most common oxidised fatty acid in LDL. Once linoleic acid becomes oxidised in LDL, aldehydes and ketones covalently bind apoB, creating LDL that is no longer recognised by the LDL receptors in the liver but is now recognised by scavenger receptors on macrophages leading to the classic foam cell formation and atherosclerosis.
- Hence, the amount of linoleic acid contained in LDL can be seen as the true ‘culprit’ that initiates the process of oxLDL formation as it is the linoleic acid that is highly susceptible to oxidation.
- Additionally, an increase in the intake of linoleic acid intake increases the linoleic acid content of very-low-density lipoprotein (VLDL) and high-density lipoprotein (HDL) increasing their susceptibility to oxidise, which further increases the risk of cardiovascular disease.
- …a more comprehensive theory, the ‘oxidised linoleic acid theory of coronary heart disease’, is as follows: dietary linoleic acid, especially when consumed from refined omega-6 vegetable oils, gets incorporated into all blood lipoproteins (such as LDL, VLDL and HDL) increasing the susceptibility of all lipoproteins to oxidise and hence increases cardiovascular risk.
But…it’s not just the omega-6 linoleic acid. Canola oil is rich in omega-3 fatty acids and is worse than soy oil in this next study:
TITLE: Differential effects of dietary canola and soybean oil intake on oxidative stress in stroke-prone spontaneously hypertensive rats.
- Article Quotes “In conclusion, canola oil ingestion shortens the life span of SHRSP rats and leads to changes in oxidative status, despite an improvement in the plasma lipids.”
- In other words, canola oil lowers LDL and that’s a BAD thing. (Again, the AHA misleads doctors into believing LDL causes heart disease and therefore lowering it is good.)
Title: “Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation”
Unsaturated fatty acids (both omega-6 linoleic acid and omega-3 alpha linolenic acid) promote inflammation of the cells lining the artery (endothelial cells)
- Article title and link: “Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells” [LINK]
- The article concludes: “Specific unsaturated dietary fatty acids, particularly linoleic acid, can selectively stimulate the development of a proinflammatory environment within the vascular endothelium.”
- Key point: The article states that the reason PUFAs promote inflammation is that they cause oxidative stress. “Although it is known that selected fatty acids can induce oxidative stress and activate transcription factors responsive to oxidative stress (13), the specific effects of unsaturated fatty acids on inflammatory responses in endothelial cells are not fully understood.” They they go on to list a number of intermediate compounds that link oxidation of PUFA to an inflammatory response
PUFA (specifically linoleic acid) cause inflammation of the cells lining the arterial (endothelial cells)
- Article link: https://www.jlr.org/content/50/2/204.short
- Article Quotes:
- TGRL lipolysis during the postprandial state releases excess FFAsin the immediate proximity of the endothelium, which may cause endothelial injury (11) and subsequent endothelial dysfunction (32).
- Therefore, although the oxidative metabolism of FFA in endothelial cells can produce inflammatory responses, TGRL lipolysis can also release preformed mediators of oxidative stress (e.g., HODEs) that may influence endothelial cell function in vivo by stimulating intracellular ROS production.
- In addition, TGRL remnants derived from TGRL lipolysis are themselves atherogenic with properties similar to oxidized LDL
- high levels of serum PUFAs, when insufficiently protected from peroxidation, may increase the risk of atherosclerotic complications (43).
- Reaven et al. (57), studying the effect of different FAs on lipoproteins, showed that only the percentage of LA in LDL was strongly associated with the extent of lipoprotein oxidization..
Title: “Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients”
LA (a PUFA) in LDL oxidizes in the bloodstream in vivo and the more oxLA products in LDL, the more atherosclerosis, therefore LDL is not inherently bad, the problem is the PUFA. As we age, we lose ability to control oxidation and get more heart attacks (on a higher PUFA diet this would happen faster than on a low pufa diet)
Article link: https://pubmed.ncbi.nlm.nih.gov/9488997/
Article quotes
9-HODE is much more abundant in oxidized LDL than other lipid peroxidation products and therefore an indicator of lipid peroxidation (LPO).
In this study the 9-HODE content in the LDL of 19 obviously healthy volunteers and 17 atherosclerotic patients was investigated.
The level of 9-HODE obtained from LDL of young atherosclerotic patients (aged 36-47 years) was increased by a factor of 20 when compared with samples from healthy volunteers of the same age group
as individuals grow older LDL becomes more and more oxidized.
Consequently, assuming that LDL oxidation is a precondition for atherosclerosis–older individuals will suffer from atherosclerosis, even if no easy detectable visible signs of this disease are recognizable.
According to 9-HODE determination, the onset of the disease starts slowly in most individuals at around 50 years of age.
Title: “HDL enhances oxidation of LDL in vitro in both men and women”
Authors find that PUFA promotes oxidation of both HDL and LDL, and HDL is more easily oxidized than LDL, which is in contrast to claims that HDL is “good” because it has an antioxidant capacity that protects LDL from oxidation.
Article link: https://lipidworld.biomedcentral.com/articles/10.1186/1476-511X-4-25
Article quotes:
Oxidation rate was positively associated with polyunsaturated fatty acid content of the lipoproteins in that it was positively related to the content of linoleate and negatively related to oleate.
More saturated fats thus protected the lipoproteins from damage.
Our findings support previous results that in vitro HDL is oxidized fastest of all lipoproteins partly because of its fatty acid composition, which is oxidation promoting.
The first phase of rapid oxidation in such a profile, whether observed with HDL or LDL, has been interpreted to occur via a tocopherol-mediated mechanism where vitamin E acts as a prooxidant.
Title “Effects of linoleate-enriched and oleate-enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans”
The more Linoleic acid )LA) a person ate, the more oxidized their circulating LDL particles, and the smaller and denser their LDL particles.
LDL is oxizable while in circulation and does not need to enter endothelial wall first
The more oxidized LDL, the less LA remains. Thus, correlating plasma LA with MI is a fools game and what needs to be tested is oxidized LA breakdown products, or oxLAMS (see study below showing these do correlate with MI)
Vitamin E supplementation does not prevent LDL oxidation when the diet is high in LA
Article quotes:
levels of lipid peroxides after 4 and 6 weeks of dietary supplementation were consistently higher in the linoleate diet group at each time point, with differences achieving statistical significance at the 4-week interval
In response to oxidation, marked decreases in the LDL content of 18:2 and 20:4 occurred in all diet groups, but a significantly greater percent loss of unsaturated fatty acids occurred in the LDL from the linoleate diet group (8% decrease in 18:l,/>=.07; 75% decrease in 18:2,P=.O6; and a 95% decrease in 20:4, P=.O5) compared with the other diet groups.
The absolute fatty acid loss due to oxidation was also significantly greater in the linoleate diet group ), whereas there was no difference in fatty acid loss between the oleate and standard-diet groups.
LDL isolated from the linoleate diet group was oxidized more rapidly and had more extensive fatty acid decomposition despite a similarly high content of a-tocopherol.
Thus, by providing LDL with more substrate for oxidation, a diet sufficiently enriched in linoleic fatty acids can overcome the antioxidant effects of high dosages of a-tocopherol.
Article quotes
Title: “Acrolein Is a Product of Lipid Peroxidation Reaction”
This article reports on original research into the primary driver of oxLDL formation. While some have proposed oxLDL forms as a function of numerous non-PUFA related factors—most notably the number of apoB particles, the amount of cholesterol or saturated fat, and glucose—support for these ideas comes only from hypothesis and not from direct evidence. This article demonstrates direct evidence that oxygen attacks the PUFA in LDL and initiates the free radical cascades leading to oxLDL. OxLDL in this case is measured by commercial assays looking for aldehyde substituted lysine residues (on ApoB).
Article link: https://www.jbc.org/content/273/26/16058.long
It has been proposed that LDL undergoes oxidative modification before it can give rise to foam cells, the key component of the progression of atherosclerosis.
It has been believed that the oxidation of LDL in vivo can be reproduced by in vitro incubation of LDL with cultured cells such as endothelial cells (6-9), smooth muscle cells (7, 10), and macrophages (11) or by auto-oxidation catalyzed by cupric ion in the absence of cells (12).
These cell-mediated or metal-catalyzed modifications convert LDL to a form that is recognized by macrophages far more readily than normal LDL (6,13).
The uptake of oxidized LDL leads to the formation of foam cells, the dominant cells in atherosclerotic lesions (4). During incubation of LDL with cells, the LDL molecule undergoes a large number of structural changes that alter its metabolism (1).
Although a detailed mechanism for the oxidative modification of LDL has not been established, it is generally accepted that the primary generation of lipid hydroperoxide derivatives initiates a reaction cascade leading to rapid propagation and to amplification in the number of reactive oxygen species formed; this ultimately leads to extensive fragmentation of the fatty acid chains (14) and conversion of the LDL to a more atherogenic form
Title: “Liposome-like particles isolated from human atherosclerotic plaques are structurally and compositionally similar to surface remnants of triglyceride-rich lipoproteins.”
This article discusses that “liposome-like particles” are found in atherosclerotic plaques that they believe are “fragments of lipoproteins.”
Supports the idea that all lipoproteins, not just LDL, can contribute to plaque formation.
Article link: https://www.ahajournals.org/doi/abs/10.1161/01.atv.14.4.622
Article quotes:
the liposome-like particles isolated from postmortem human atherosclerotic plaques are rich in intact apolipoprotein (apo) A-I, C apolipoproteins, and sphingomyelin.
We propose as an alternate explanation that in vivo liposome-like particles are lipolytic surface remnants of TG-rich lipoproteins. We further suggest that these remnants are produced in the intimal space by undefined processes and/or are transcytosed into the intima from the plasma compartment as a product of normal lipolysis gone awry.
Title: “Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein(a)”
Human trial showing that even on a low fat diet and when comparing relatively low dietary PUFA levels, the higher PUFA intake correlates with greater LDL oxidation and increased Lp(a). Lp(a) is soemtimes used as a ‘poor man’s’ marker of oxidized LDL.
https://pubmed.ncbi.nlm.nih.gov/14739118/
Article quotes:
The dietary intake of total fat was 70 g per day at baseline and decreased to 56 g (low-fat, low-vegetable diet) and to 59 g (low-fat, high-vegetable diet).
The saturated fat intake decreased from 28 g to 20 g and to 19 g, and the amount of polyunsaturated fat intake increased from 11 g to 13 g and to 19 g (baseline; low-fat, low-vegetable; low-fat, high-vegetable; respectively).
The amount of oxidized LDL in plasma was determined as the content of oxidized phospholipid per ApoB-100 using a monoclonal antibody EO6 (OxLDL-EO6).
The median plasma OxLDL-EO6 increased by 27% (P<0.01) in response to the low-fat, low-vegetable diet and 19% (P<0.01) in response to the low-fat, high-vegetable diet. Also, the Lp(a) concentration was increased by 7% (P<0.01) and 9% (P=0.01), respectively.
Title: “Oxidized fatty acids promote atherosclerosis only in the presence of dietary cholesterol in low-density lipoprotein receptor knockout mice.”
Eating fried high-PUFA oils oxidizes LA but oxLA are not found in LDL, therefore oxLA promotes LDL oxidation indirectly, likely by depleting antioxidant capacity.
Article quotes
We speculate that the proatherogenic effects of oxidized fatty acids in the high-fat diet group may arise by two mechanisms. First, dietary oxidized fatty acids may directly or indirectly increase oxidative stress and the presence of oxidized LDL and other lipoproteins in the plasma and arterial walls, thereby initiating fatty streak formation. It has been previously shown that there is a 10-fold increase in the uptake of chylomicrons by macrophages after consumption of heated oil.
15% of PUFAbecome oxidized in the process of preparing french-fried potatoes. Therefore, a medium serving of french fries, which contains 15 g of fat, could contain 2.3 g of oxidized fatty acids.
We fed 8 mg of 13-HODE to the mice daily, which in humans is comparable with consumption of less than half of a medium serving of french fries daily. This level of oxidized fatty acid consumption is quite reasonable considering the Western diet that is commonly consumed
Our study suggests that even small quantities of oxidized linoleic acid promotes an athero-genic lipoprotein profile and atherosclerosis in the presence of a cholesterol-rich diet.
People with higher dietary fat and cholesterol intakes usually consume more fried foods.
Title: “Lipid hydroperoxy and hydroxy derivatives in copper-catalyzed oxidation of low density lipoprotein”
When LDL oxidizes, its PUFA content goes down, having been oxidized into different compounds. This confounds studies correlating plasma PUFA with heart disease. You need to measure oxLAMS not PUFA to do useful research.
Aricle Link: https://www.jlr.org/content/31/6/1043.full.pdf+html
Article quotes
Oxidized LDL showed decreased linoleate (18:2) and arachidonate (20:4) content with increased concentrations of thiobarbituric acid reactive substances (TBARS) and hydroxy and hydroperoxy 18:2 and 20:4. The electrophoretic mobility of the LDL protein also was increased by oxidation.
Aldehydes, through Schiff base formation with amino acid may lead to increased electronegativity of LDL and enhanced affinity for scavenger receptors on macrophages
Title: “Dietary polyunsaturates and peroxidation of low density lipoprotein.”
Omega-3 promotes oxLDL also, not just an omega-6 issue
Article link: https://pubmed.ncbi.nlm.nih.gov/8925192/
Article quotes
Omega-6 polyunsaturated fatty acids enhance the susceptibility of low density lipoprotein to oxidation compared with monoenes.
Most studies on omega-3 fatty acids also exhibit increased peroxidation of low density lipoprotein, although these data are more conflicting.
Long-term dietary habits predict the fatty acid composition of serum and LDL, and influence the susceptibility of LDL to oxidation.
In the fish group with the highest content of omega-3 fatty acids in LDL, the oxidation susceptibility of LDL was highest.
In the vegetarian group with less omega-3 fatty acids in LDL, the LDL was more resistant to oxidation.
Title: “Effects of oleate-rich and linoleate-rich diets on the susceptibility of low density lipoprotein to oxidative modification in mildly hypercholesterolemic subjects.” (from 1993, UC San Diego)
Takeaways:
Diets high in PUFA promote atherosclerosis because PUFA promote LDL oxidation and LDL oxidation promotes foam cell formation and atherosclerosis. Without PUFA oxidation you don’t get oxidized LDL.
Without PUFA oxidation you don’t get oxidized LDL
PUFA reduce the oxidative benefits of HDL and lower HDL counts
Takes 5 weeks after diet change (to increased PUFA) to see the oxidative effects on LDL and changes in HDL/triglycerides
They do not study omega-3 18:3
Diets high in PUFA reduce LDL AND HDL because these particles oxidize. This is not a good thing.
Article link: Https: //www.ncbi.nlm.nih.gov/pmc/articles/PMC288008/
Article Quotes
“the polyunsaturated fatty acids in the phospholipid surface are the first to undergo lipid oxidation. Subsequently, lipid oxidation extends into the LDL core where extensive loss of polyunsaturated fatty acid occurs.”
“The oleic acid present in the LDL phospholipid fraction, whether isolated from individuals consuming oleate-enriched, linoleate-enriched, or control diets, was virtually unchanged during prolonged oxidation.”
“The generation of oxidatively modified LDL appears dependent on the peroxidative decomposition of its polyunsaturated fatty acids, yielding reactive aldehydes some of which form covalent bonds with apo B generating a modified LDL recognized by the scavenger receptors of the macrophage:”
“Diets enriched in polyunsaturated fatty acids are considered to be beneficial because of their hypocholesterolemic effects. However, these diets lead to LDL particles enriched in polyunsaturated fatty acids, which should be more susceptible to lipid peroxidation and in principle possibly more atherogenic.
Title: “Endogenous cholesterol ester hydroperoxides modulate cholesterol levels and inhibit cholesterol uptake in hepatocytes and macrophages”
This article’s title totally buries the lead. Their findings show that the lipid biomarker most closely associated with heart attacks is cholesterol that’s bonded to hydroperoxide of linoleic acid. So-called “endogenous cholesterol ester hydroperoxide” levels were elevated in the bloodstream of people with recent MI, and only those with recent MI had measurable levels of plasma cholesterol esterified to oxidized linoleic acid. This blows out of the water the ideas that 1. PUFAs don’t oxidize in circulating LDL and that 2. LDL is inherently bad. It’s not LDL. It’s PUFA.
Article Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6302155/
More takeaways:
It remains to be seen if these compounds are present before MI; this study evaluated people who’d already had heart attacks and the authors point out that while it’s tempting to assume they were present beforehand, these compounds may be present only as result of the oxidation induced by MI.
What we do know is
People without MI were also without oxidized-PUFA (oxlam) cholesterol esters. See this article’s figure 1 below.
In those with MI in this study, high LDL appeared to be a marker of LDL oxidation.
The reason for high LDL in this group of people is that oxidized PUFA esterified to cholesterol in LDL can downregulate the production of LDL receptors on the liver and WBCs, thus keeping LDL in circulation longer.
Figure 1 from the article has a caption that reads: The levels of oxCEs were significantly elevated in plasma of patients with MI. This supports the idea that PUFA oxidizes LDL and then and only then will LDL promote MI. Abbreviations: LA-CE = Linoleic acid cholesterol ester, AA-CE = Arachidonic acid cholesterol ester, CEOOH = cholesterol ester hydroperoxide, CEOH = cholesterol ester peroxide
Article title: “The role of dietary oxidized cholesterol and oxidized fatty acids in the development of atherogenesis”
Takeaways: Seed oils used for frying are high PUFA. Partially oxidized PUFA products and oxidized cholesterol from deep-fried foods are absorbed into the body. Both are incorporated into chylomicrons but only the oxidized cholesterol is found in apoB containing lipoproteins. Therefore any oxidized PUFA found in apo-B containing lipoproteins would be produced in the body.
Article link: https://pubmed.ncbi.nlm.nih.gov/16270280/
Article quotes:
A typical Western diet is rich in oxidized fats and therefore could contribute to the increased arterial atherosclerosis in our population
We hypothesize that diet-derived oxidized fatty acids in chylomicron remnants and oxidized cholesterol in remnants and LDL accelerate atherosclerosis by increasing oxidized lipid levels in circulating LDL and chylomicron remnants.
Article Title: “Effects of antioxidants and fatty acids on low-density-lipoprotein oxidation”
Link: https://pubmed.ncbi.nlm.nih.gov/7977141/ (Abstract)
Takeaway: Reducing dietary PUFA is likely to prevent atherosclerosis by preventing LDL particle oxidation. Supporting quotes:
- Supplementation of animals and humans with antioxidants such as alpha-tocopherol have shown promise in reducing the extent of LDL oxidation.
- However, another possible means of preventing LDL oxidative modification may be by reducing the amount of oxidizable polyunsaturated fatty acids in the LDL particle.
Asthma
Background: Asthma is a disease of lung inflammation. Lung inflammation can be helpful–when it’s kept in check. The purpose of lung inflammation is to eliminate toxins and microbes from the airway. When you have asthma, triggers like dander, pollen, or viruses kick off an inflammatory cascade that makes you cough and produce mucus. This reaction is designed to eliminate the triggering agent. But when we’ve lived on vegetable oils, our bodies are chronically depleted of antioxidants. Without antioxidants, our body cannot quickly turn off inflammation. Steroids help to stop some of the oxidation, and people with asthma often need to take steroid drugs to calm their asthma attacks.
I have had numerous patients experience relief from making the dietary changes I advise, which focus on avoiding the Hateful Eight vegetable oils and avoiding soda, juice, and other sugary junk. Complex carbs, however, are ok by me.
The most top-of-mind success story is a child who went from being diagnosed with “severe refractory asthma” and having repeated life-threatening attacks (so much so that her parents moved to live closer to the hospital), to having 1 or 2 attacks a year when she gets a cold or flu, and those are managed at home.
Evidence of the direct link between asthma and PUFA oxidation is that we treat asthma attacks with corticosteroids. Corticosteroids block the formation of inflammatory eicosanoids by blocking COX enzymes that activate inflammation. COX stands for cyclo-oxygenase, and it oxidizes PUFAs. Both omega 3 and omega 6. Once this oxidation is started it is very difficult to control when a person’s body is depleted of compounds like glutathione that turn off the inflammatory responses.
Other support: Asthmatics have lower glutathione levels and higher levels of all measured lipid oxidation products, both omega-3 and omega-6.
This article describes a study in children where the investigators found asthmatics have more biomarkers of lipid oxidation during attacks (the subset called peroxidation) than when quiescent, and more than controls, and gives you some background.
These studies have linked PUFA to asthma.
“Linoleic acid metabolite drives severe asthma by causing airway epithelial injury”
- Link: https://www.nature.com/articles/srep01349
- Comment: The article title implies that this is part of a metabolic pathway, in other words, something the body does on purpose. However, the body cannot control the reactions between oxygen and linoleic acid, and, as the article states, those reactions also produce a significant amount of injurious PUFA metabolites. Likely, the majority since these non-enzymatic reactions are orders of magnitude faster.
- It matters that these toxins are not generated on purpose by enzymes. Why? Because if the conclusion is that the body does this on purpose, then your genes will be blamed for inflammation caused by seed oils.
“Lipid mediator profiles differ between lung compartments in asthmatic and healthy humans”
- Link: https://erj.ersjournals.com/content/43/2/453
- Comment: The term “lipid mediator” refers to oxidation products of linoleic acid that trigger an over-exuberant inflammatory response in the lungs, which is the hallmark of asthma. The article supports my theory that too much PUFA depletes antioxidants and this prolongs the inflammatory response.
Blood clots
Rapeseed oil and sunflower oil diets enhance platelet in vitro aggregation and thromboxane production in healthy men when compared with milk fat or habitual diets
https://pubmed.ncbi.nlm.nih.gov/1641826/
Brain development abnormalities
Linoleic acid excess causes abnormal brain development
Linoleic acid–good or bad for the brain? [Ameer Taha, author]
Pre-clinical evidence suggests that excess dietary LA increases the brain’s vulnerability to inflammation and likely acts via its oxidized metabolites.
In humans, excess maternal LA intake has been linked to atypical neurodevelopment, but underlying mechanisms are unknown.
It is concluded that excess dietary LA may adversely affect the brain. The potential neuroprotective role of reducing dietary LA merits clinical evaluation in future studies.
Cancer
Human Study Linking Dietary PUFA to Oxidative Stress and DNA Damage Seen in Cancer
TITLE: Influence of dietary fatty acid, vegetable, and vitamin intake on etheno-DNA adducts in white blood cells of healthy female volunteers: a pilot study
LINK https://pubmed.ncbi.nlm.nih.gov/11700267/
Basic Mechanism is Oxidative Stress
“Mitochondrial Dysfunction in Cancer and neurodegenerative Diseases: Spotlight on Fatty AcidOxidation and Lipoperoxidation Products”
When mitochondria burn PUFA for energy this produces oxidative stress, membrane damage and the free radicals and other oxidation products that can damage DNA.
Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers
https://academic.oup.com/carcin/article/20/12/2209/2529842
“persistent oxidative stress, often involving enhanced peroxidation of PUFAs in cell membranes by intracellularly produced O – and N -centred free radicals, altered cellular redox potential, activation of protein kinases and subsequent changes in transcription factors, are now known to enhance the development of malignant diseases.”
“Thus, the carcinogenic process could be initiated and/or accelerated by lipid peroxidation-induced DNA and protein damage.”
Free-radicals and advanced chemistries involved in cell membrane organization influence oxygen diffusion and pathology treatment
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5707132/
Posits that PUFA oxidation is a major factor in cancer development. Explains that cancer cells have irregular appearing warped membranes compared to healthy cells because PUFAs promote cell membrane polymerization.
Cancer Types
LUNG CANCER from inhaling deep fryer fumes
- Effects on Chinese Restaurant Workers of Exposure to Cooking Oil Fumes: A Cautionary Note on Urinary 8-Hydroxy-2?-Deoxyguanosine.
- Mechanism: PUFA deteriorates into volatile toxins, including acrylamide, which is a known potent carcinogen.
- Read Article https://aacrjournals.org/cebp/article/17/12/3351/66983/Effects-on-Chinese-Restaurant-Workers-of-Exposure
Crohn’s Disease
“Lipid and Bile Acid Dysmetabolism in Crohn’s Disease”
A recent systematic review of 19 studies reported that high intakes of total fats, total polyunsaturated fatty acids (PUFAs), n-6 PUFAs, and meat were associated with an increased risk of Crohn’s disease
Diabetes (See Insulin Resistance and Diabetes)
Endothelial Dysfunction
“Linoleic Acid Increases Lectin-Like Oxidized LDL Receptor-1 (LOX-1) Expression in Human Aortic Endothelial Cells”
This article shows that linoleic acid increases endothelial disfunction and inflammatory marker expression. It also asserts that diabetics have more linoleic acid in their LDL particles than nondiabetics. It is an in vitro experiment.
https://diabetes.diabetesjournals.org/content/54/5/1506.long#ref-13
Article quotes
Results from in vitro studies suggest that selected fatty acids, and especially linoleic acid (LA), can elicit endothelial dysfunction (ED).
LA is increased in all LDL subfractions in patients with type 2 diabetes, this alteration may contribute to ED associated with diabetes
Lectin-like oxidized LDL receptor-1 (LOX-1) is the major endothelial receptor for oxidized LDL (oxLDL), and uptake of oxLDL through LOX-1 induces ED
Fatty Liver
Oxidized metabolites of linoleic acid as biomarkers of liver injury in nonalcoholic steatohepatitis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4578804/
Article quotes:
Recent studies have uncovered the importance of certain components of the diet. In this review, we will focus on the growing evidence for a central role of n-6 polyunsaturated fatty acids.
We will discuss novel findings linking oxidative stress and increased production of reactive oxygen species in the liver to oxidation of n-6 polyunsaturated fatty acids and production of specific lipid oxidation metabolites.
In particular, we will highlight the potential role of these metabolites as noninvasive markers to diagnose and monitor the extent of liver damage in patients with NAFLD.
linoleic acid oxidation generates cytotoxic compounds that profoundly affect liver metabolism, damage and kill liver cells, and cause fatty liver. They also promote liver insulin resistance (see heading for insulin resistance).
From “Influence of total polar compounds on lipid metabolism, oxidative stress and cytotoxicity in HepG2 cells”
Oxidized PUFA kill liver cells (“reduce cell survival”)
Excessive intake of free fatty acids by HepG2 cells should active lipogenesis-related enzymes, which further promote the production of intracellular lipid [25, 26]. We found TPC could significantly induce lipid accumulation in HepG2 cells and reduce the cell survival, corresponding to the behavior of oxidized oil
Gut Inflammation and Colitis
PUFA Promote Ulcerative Colitis
“Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study”
Shows that: High intake of LA more than doubled the risks of IBD and could be responsible for ~30% of ulcerative colitis cases. Quote “The data support a role for dietary linoleic acid in the aetiology of ulcerative colitis. An estimated 30% of cases could be attributed to having dietary intakes higher than the lowest quartile of linoleic acid intake”
PUFA Promote Crohn’s Disease
“Lipid and Bile Acid Dysmetabolism in Crohn’s Disease”
A recent systematic review of 19 studies reported that high intakes of total fats, total polyunsaturated fatty acids (PUFAs), n-6 PUFAs, and meat were associated with an increased risk of Crohn’s disease
PUFA Promote Gut Inflammation and Oxidative Stress
“Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: role of intestinal absorption of 4-HHE and reactivity in intestinal cells.”
Consumption of oxidized n-3 PUFA results in 4-HHE accumulation in blood after its intestinal absorption and triggers oxidative stress and inflammation in the upper intestine.
In conclusion, our study demonstrates in mice that long-term ingestion of weakly oxidized n-3 PUFA induces an accumulation of 4-HHE in plasma, which can be partly due to 4-HHE absorption in the small intestine.
Furthermore, weakly oxidized n-3 PUFA enhance inflammatory markers in plasma and ER stress in the upper small intestine and reduce Paneth cell number in the duodenum, possibly through the biological reactivity of 4-HHE.
GPx2 appears to be required to defend against injury by oxidative stress markers and prevent inflammation in the intestine. The upregulation of GPx2 and GRP78 can be considered as an attempt to counteract increased inflammation and oxidative stress, noticed by the formation of Michael adducts and reactive carbonyls on intestinal proteins.
Altogether, we highlight that the potential harmful effects of dietary oxidized n-3 PUFA, even if consumed in moderate doses, should be taken into account in several pathologies. It appears of utmost importance in medical and nutritional practice to formulate and handle n-3 PUFA-rich foods or supplements with the greatest care to avoid any PUFA oxidation. This can be particularly relevant in patients suffering from acute or chronic diseases who increase their n-3 PUFA intake because of their reported health benefits.
Gut Microbiome Abnormalities
Longitudinal Effects of Dietary Oxidized Lipids on the Gut Microbiome and Mycobiome in PigLongitudinal Effects of Dietary Oxidized Lipids on the Gut Microbiome and Mycobiome in Pigs
https://faseb.onlinelibrary.wiley.com/doi/10.1096/fasebj.2020.34.s1.09676
“we demonstrate that dietary LOPs can alter the bacterial and fungal communities in the gut, even when controlling for total dietary fat intake. These findings suggest that the oxidation state of dietary lipids is an important factor to consider when studying the effects of western diets on chronic disease risk.’ LOP= lipid oxidation products
Resilience of small intestinal beneficial bacteria to the toxicity of soybean oil fatty acids
https://elifesciences.org/articles/32581
Linoleic acid and the other two major unsaturated FAs in SBO, oleic acid (18:1), and alpha-linolenic acid (18:3), are known to be bacteriostatic and/or bactericidal to small intestinal bacteria as non-esterified (free) fatty acids in vitro at concentrations found in the small intestine, It is interesting to note that the human-associated L. reuteri underwent a population bottleneck that coincides with the increase in SBO consumption in the U.S. and is far less prevalent than it was in the past (Walter et al., 2011). Despite its decline, L. reuteri and other lactobacilli persist in the small intestine of Western individuals, suggesting mechanisms to counter the inhibitory effects of FAs in vivo.
Heart Attack (see Atherosclerosis)
Heart Failure
Diastolic Dysfunction
Article Title: “Excess Linoleic Acid Increases Collagen I/III Ratio and “Stiffens” the Heart Muscle Following High Fat Diets”
Article quotes:
Corn oil diet induces changes to cardiac fatty acids and causes early diastolic dysfunction without altering systolic function
CO feeding led to a three-fold increase in cardiac LA over 5 weeks compared to OO fed hearts (Abbv. Corn Oil, Olive Oil, Linoleic Acid)
HDL Dysfunction and lowered HDL
Omega-3 reduce anti-inflammatory capacity of HDL
“Polyunsaturated fatty acids supplementation impairs anti-oxidant high-density lipoprotein function in heart failure.”
After 12 weeks of treatment, the anti-oxidant function of HDL, measured by the HDL inflammatory index, was found significantly impaired in the treatment group in a dose-dependent fashion. [source of n3 not clarified if c18 or higher)
The underlying pathophysiological mechanisms remain unclear but may include pro?oxidant effects of n3?PUFA.
As apolipoprotein A1, the major constituent of HDL is significantly synthesized by the upper intestine, and one may speculate that the pro?oxidant intestinal environment could impair the protein quality
Omega-3 Reduce HDL
“Dietary n-3 polyunsaturated fat increases the fractional catabolic rate of medium-sized HDL particles in African green monkeys.”
diets enriched in n-3 poly fat result in lower plasma HDL cholesterol and apoA-I concentrations than do diets enriched in saturated fat
In the present study, in which factors that determine individual responsiveness to an atherogenic diet were carefully controlled, dietary fat affected the fractional catabolic rate (FCR) of apoA-I.
In animals fed the n-3 poly diet, the apoA-I FCR for medium HDL was increased approximately threefold compared with the Sat group. This increased FCR explains the reduced plasma concentration of medium HDL particles in the n-3 poly group that was observed despite the similar production rate in the two diet groups. The n-3 poly animals also had increased FCR values for large and small HDL compared with the Sat animals; however, this increase was not statistically significant due to the heterogeneity of the FCR values in the groups.
PUFA reduce HDL (when they replace carb)
Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials.
https://www.ahajournals.org/doi/pdf/10.1161/01.ATV.12.8.911
Replacing 1% of carb with sat, mono and PUFA has predictable changes on HDL, Trig, LDL and TCHOL.
See IMAGE BELOW
PUFA reduce HDL
Insulin Resistance and Diabetes
Hypothesis: Mitochondria burning PUFA cause extensive oxidative stress and harmful free radicals/ROX. This causes the cell to down regulate the use of fatty acids to produce ATP and upregulate glycolysis. This is the Warburg effect. And in some people, glycolysis is upregulated in so many cells that their body’s glucose requirement increases that in response, their glucose setpoint is increased. In this case, the new, higher setpoint is regulated by the brain, which instructs the liver meet demand by accellerating gluconeogenesis. Thus fasting blood sugar goes up. This is the beginning of insulin resistance because the pancreas produces insulin to reduce blood sugar to normal, and the liver must ignore that in order to continue its accelerated pace of gluconeogenesis.
1995 Hypothesis Paper Links Insulin Resistance to PUFA
“Is insulin resistance influenced by dietary linoleic acid and trans fatty acids?” By Artemis Simopoulos
Article Quote
Recent findings that C20-C22 in muscle membrane phospholipids are inversely related to insulin resistance, whereas linoleic acid is positively related to insulin resistance, suggest that diet may influence the development of insulin resistance in obesity, insulin-dependent diabetes mellitus (IDDM), hypertension, and coronary artery disease (including asymptomatic atherosclerosis and microvascular angina).
PUFA cause oxidative stress, which causes cellular glucose dependence [The Warburg Effect]
“Mitochondrial Dysfunction in Cancer and Neurodegenerative Diseases: Spotlight on Fatty Acid Oxidation and Lipoperoxidation Products.”
Article Quote
It has been demonstrated that the Warburg effect is also present in normal, highly proliferating cells, in which the increase of aerobic glycolysis, associated with a reduced OXPHOS activity, could be a protective strategy against ROS
PUFA Infusions Acutely Elevate Insulin Level (Linoleic acid, specifically)
“The Natural PPAR Agonist Linoleic Acid Stimulated Insulin Release in the Rat Pancreas”
Article Quotes
Linoleic acid alone stimulated a 391% increase in the peak insulin concentration compared with the baseline in the rats fed a normal diet.
The peak insulin concentration decreased significantly to183% in the rats fed a long-term high-fat diet.
All the results suggested that unsaturated fatty acids stimulated insulin secretion and additively increased glucose-induced insulin secretion in the perfused rat pancreas.
Diabetes
Singlet Oxygen Induced Products of Linoleates, 10- and 12-(Z,E)-Hydroxyoctadecadienoic Acids (HODE), Can Be Potential Biomarkers for Early Detection of Type 2 Diabetes
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3655182/
We found that the fasting levels of (10- and 12-(Z,E)- HODE)/LA increased significantly with increasing levels of HbA1c and glucose during OGTT and with insulin secretion and resistance index.
The fatty acid distribution in low density lipoprotein in diabetes
https://pubmed.ncbi.nlm.nih.gov/10395970/
Diabetics have increased PUFA (LA) in LDL compared to non diabetics.
“There is an association between small dense low density lipoprotein (LDL) phenotype, which is more prevalent in the diabetic state, and atherosclerosis. Small dense LDL is more easily oxidised and it is possible that fatty acid compositional changes, particularly an increase in polyunsaturated fatty acids, could underlie this association. ‘
PUFA Promotes Insulin Resistance more than sat fat [both n3&6]
High fat diet induced IR
Rats fed this chow https://researchdiets.com/formulas/d12266b
Roughly 45% of fat calories are PUFA (30% fat is high fat for rats apparently)
“Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice.”
“Skeletal muscle insulin resistance is induced by 4-hydroxy-2-hexenal, a by-product of n-3 fatty acid peroxidation.” https://www.ncbi.nlm.nih.gov/pubmed/29299636
Circulating levels of 4-HHE in type 2 diabetic humans … were twice those in their non-diabetic counterparts (33 vs 14 nmol/l, p?<?0.001), and positively correlated with blood glucose levels
4-HHE therefore plays a causal role in the pathophysiology of type 2 diabetes and might constitute a potential therapeutic target to taper oxidative stress-induced insulin resistance.
? why is 4HHE higher in DM? Does it reflect body fat pufa?
“A peroxidized omega-3-enriched polyunsaturated diet leads to adipose and metabolic dysfunction.” https://www.ncbi.nlm.nih.gov/pubmed/30439568
While healthful, n-3 fats are prone to peroxidation, and we observed peroxidated lipid metabolites in the adipose tissue of mice on these diets.
The brain remained mostly shielded from changes in dietary fat type and amount, but differences in adipose lipid metabolites across these six diets may have affected metabolic function and brain-adipose communication, as observed in this study.
“4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance.” (4HNE comes from n-6 PUFA)
https://www-ncbi-nlm-nih-gov.prx.hml.org/pubmed/28088621
Increased adipose production of 4-hydroxynonenal (4-HNE), a bioreactive aldehyde, directly correlates with obesity and insulin resistance.
Obesity-associated insulin resistance is marked by impaired SC [sub Q fat] adipogenesis while IS [Insulin senstitive] obese individuals exhibit a more efficient adipogenesis . This contributes to the expansion of visceral fat in IR obese subjects with ectopic fat deposition in the liver, skeletal muscle and other depots such as epicardial fat, causing further augmentation of IR in these tissues.
The cellular concentration of 4-HNE varies from 0.1 to 0.3 µM under physiological conditions and from 10 µM up to 5 mM under oxidative stress. Differences in the redox status and increased 4-HNE levels of human adipose tissues in relation to obesity and metabolic risk were previously reported [17]. This elevation can progressively impair cell structure and function due to the accumulation of stable adducts formed with proteins, phospholipids and DNA. The generation of high levels of 4-HNE has been associated with inflammatory processes through the activation of cyclooxygenase, under pathological conditions such as insulin resistance, atherosclerosis and obesity
In the present study, we hypothesize that in vitro long-term exposure of SubCutaneous preadipocytes from IS individuals to 4-HNE can impair their adipogenesis and insulin sensitivity, rendering their behavior like that seen in preadipocytes from IR individuals.
In conclusion, this study provides novel evidence for 4-HNE-mediated inhibition of human preadipocytes growth, impairment of adipogenesis and induction of insulin resistance, thus highlighting its crucial role in the progression obesity-associated metabolic syndrome. The data also identifies 4-HNE as a key player in the pathophysiology of adipose tissue in IS obesity and potentially a therapeutic target before progression into IR obesity.
“Influence of total polar compounds on lipid metabolism, oxidative stress and cytotoxicity in HepG2 cells”
Recently, the harmful effects of frying oil on health have been gradually realized. However, as main components of frying oils, biochemical effects of total polar compounds (TPC) on a cellular level were underestimated.
Recently, the harmful effects of frying oil on health have been gradually realized. It was reported that deep-fried oils and oxidized fat may take an unshirkable responsibility for inducing metabolic syndrome [4, 5].
For instance, frying oils could disturb liver function through inhibiting growth rate, promoting liver enlargement and attenuating detoxification enzymes related with defense mechanisms against in vivo lipid peroxidation [6, 7].
In addition, frying oil and its polar parts could potentially cause deformities in pregnant mice and perturbation of the vitamin A metabolic genes expression in fetal liver [8]. To our knowledge, however, the biochemical effects of TPC [total polar compounds] (the main components of frying oils) on a cellular level were underestimated.
Omega-3 produce more insulin resistance than omega-6 and control
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3180158/
Abdominal adiposity, insulin and bone quality in young male rats fed a high-fat diet containing soybean or canola oil
Study compared 19g soy versus 19g canola versus 7g soy (control, with more carbs & protein but fewer calories)
Both caused higher abdominal fat, with 19C fat cells being normal sized and more numerous and 19S being larger in size and of normal number
19C group showed significantly higher concentrations of glucose (+15%) and insulin (+56%) and an increased insulin resistance index (+78%) compared to the 19S and 7S group (see table 2, below)
Macular Degeneration
The most common cause of blindness in the developed world.
Article: “Dietary Fat and Risk for Advanced Age-Related Macular Degeneration” [jama 2001]
PUFAs promote oxidative stress in the macula
- Article Link: https://jamanetwork.com/journals/jamaophthalmology/fullarticle/267470
- Article Quotes:
- Polyunsaturated fat is an important protective factor for CVD,8 but we observed a positive association between AMD and polyunsaturated fat intake in our data.
- It is possible that high intake of polyunsaturated fat may increase unsaturation of lipids in the macula and hence increase oxidative damage.
- We found no association between exudative AMD and dietary cholesterol, animal fat, and saturated fat, which are related to CVD.
- Our results also suggest an association between AMD and higher intake of vegetable, monounsaturated, and polyunsaturated fats and linoleic acid.
- Although the mechanism for an association with AMD is not entirely clear, foods with higher levels of these types of fat overall tend to be highly processed, store-bought snack foods.
- Such foods might contain products that adversely affect blood vessels supplying the choroid or retina.
- These and other similar foods might also increase oxidative damage in the macula, which is susceptible to oxidation due to high oxygen tension in the presence of light exposure.
- This study raises the possibility that some individual dietary fats, not necessarily total fat consumption, increase or decrease the risk for advanced macular degeneration
Article: “Drusen proteome analysis: An approach to the etiology of age-related macular degeneration” [2002]
- Article link: https://www.pnas.org/content/99/23/14682.short
- Article Quotes
- Up to 65% of the proteins identified were found in drusen from both AMD and normal donors.
- However, oxidative protein modifications were also observed, including apparent crosslinked species of tissue metalloproteinase inhibitor 3 and vitronectin, and carboxyethyl pyrrole protein adducts.
- Carboxyethyl pyrrole adducts are uniquely generated from the oxidation of docosahexaenoate-containing lipids.
- By Western analysis they were found to be more abundant in AMD than in normal Bruch’s membrane and were found associated with drusen proteins.
- Carboxymethyl lysine, another oxidative modification, was also detected in drusen.
- These data strongly support the hypothesis that oxidative injury contributes to the pathogenesis of AMD and suggest that oxidative protein modifications may have a critical role in drusen formation.
Title: “A new perspective on lipid research in age-related macular degeneration”
- Article Link: https://www.sciencedirect.com/science/article/pii/S1350946217301271#bib204
- Quotes:
- The RPE is susceptible to chronic oxidative stress, which may play a role in AMD pathogenesis (Klein et al., 2014b).
- It has been suggested that oxidized lipids found in Bruch’s membrane and the RPE may be the first process leading to inflammation in AMD (Schutt et al., 2003; Spaide et al., 1999).
- …it was suggested that endogenous and dietary antioxidants might be crucial for the defense against damaging free radicals (Age-Related Eye Disease Study 2 Research Group, 2013; Age-Related Eye Disease Study Research Group, 2001).
- Antioxidants counteract lipid peroxidation either by hindering or scavenging reactive oxygen species or by stalling radical chain propagation (Laguerre et al., 2007).
- Dietary nutrient intake showed significant improvement on the defence against AMD (Preedy, 2014).
- In the future it will be important to consider the multidimensional nature of the diet and the patterns of nutrition locally and globally for AMD.
- It is likely that further observational studies and clinical trials are needed to better understand and characterize the role of nutrition, particularly PUFAs role in AMD prevention.
Title: “The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration” 2011
- Article link: https://www.researchgate.net/publication/275025345_The_role_of_oxidative_stress_and_antioxidants_in_the_pathogenesis_of_age-related_macular_degeneration
- Findings from Table 1:
- Evidence of antioxidant capacity depletion and oxidative product accumulation consistent with oxidative stress induced by PUFAs:
- Antioxidant enzymes SOD and GDx are lower in AMD patients versus matched controls (5 versus 10 and 13 versus 16 u/mL in serum).
- Markers of oxidative stress MDA and Advanced oxidation protein products–AOPP (6.4 versus 4.9 and 252 versus 181 nanomole and micromole/liter respectively).
- Glutathione is lower (310 verus 331 nmol/mL).
- Vitamin C is lower (2.5 versus 3.1 micromole/liter)
- Article quotes
- The retina is particularly susceptible to oxidative stress due to its high concentration of oxygen, its high proportino of polyunsaturated fatty acids, and its exposure to visible light.
- Serum MDA is the end product of PUFA oxidation [paraphrased this part, the english is poor] “…and thus serves as a reliable marker of oxidative stress.”
- Because the retina suffers oxidative damage, antioxidant nutrients are thought to be protective of the retina through their function as antioxidants.
- The results of the present study suggest that reductions in the antioxidant defense system and increased oxidative stress may play a role in the pathogenesis of AMD.
Title: “Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids” 2012
- Article Link:https://www.pnas.org/content/109/34/13757.short
- Article Quotes:
- It has been postulated for some time that oxidative stress plays a critical role in AMD pathogenesis (9, 10). T
- The outer segments of retinal photoreceptors possess the highest content of poly-unsaturated fatty acids (PUFAs) in the body, and these PUFAs undergo rapid turnover once every 2 wk (25, 26), releasing a large amount of PUFA-containing membrane fragments.
- These PUFAs are subject to strong oxidative stress because of exposure to high oxygen content in the outer retina and to sunlight, often exacerbated by smoking.
- It is possible that a significant amount of oxidation products such as oxPL-containing lipids or protein adducts are taken up by the RPE, presumably through receptors such as CD36.
- These products are strong inflammatory stimulants and monocyte chemoattractants(Fig. 2A), and they attract and recruit circulating monocytes and promote resident macro-phage differentiation.
- These oxidation products also can stimulate macrophages to express CD36, leading to phagocytosis of oxidized lipids and proteins.
- The oxidized lipid and protein-filled macrophages may further exacerbate inflammation, similar to the action of those described in our in vitro experiments
(PDF) Complement Factor H Genotypes Impact Risk of Age-Related Macular Degeneration by Interaction with Oxidized Phospholipids. Available from: https://www.researchgate.net/publication/230638678_Complement_Factor_H_Genotypes_Impact_Risk_of_Age-Related_Macular_Degeneration_by_Interaction_with_Oxidized_Phospholipids [accessed Jan 26 2021]. - Oxidative stress plays an important role in many aging diseases, including cardiovascular disease and AMD. A large amount of oxidized phospholipids (oxPLs) are generated in the eye because of sunlight exposure and high oxygen content.
- OxPLs bind to the retinal pigment epithelium and macrophages and strongly activate downstream inflammatory cascades.
- We hypothesize that CFH may impact the risk of AMD by modulating oxidative stress. Here we demonstrate that CFH binds to oxPLs. The CFH 402Y variant of the protective rs1061170 genotype binds oxPLs with a higher affinity and exhibits a stronger inhibitory effect on the binding of oxPLs to retinal pigment epithelium and macrophages.
- In addition, plasma from non-AMD subjects with the protective genotype has a lower level of systemic oxidative stress measured by oxPLs per apolipoprotein B (oxPLs/apoB). We also show that oxPL stimulation increases expression of genes involved in macrophage infiltration, inflammation, and neovascularization in the eye.
- OxPLs colocalize with CFH in drusen in the human AMD eye.
- Subretinal injection of oxPLs induces choroidal neovascularization in mice.
- Together, these findings suggest that CFH influences AMD risk by modulating oxidative stress, inflammation, and abnormal angiogenesis.
Title: “Dietary Saturated Fatty Acid Intake and Early Age-Related Macular Degeneration in a Japanese Population” 2020
The “Dietary Saturated Fatty Acid Intake” in Japanese article proves wrong the statement “There are currently no longitudinal prospective studies that investigated systemic plasma or serum SFA levels with AMD progression.” from this article “Risk factors for progression of age?related macular degeneration” available here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7155063/
Risk factors for progression has a nice set of images showing the progression and a section on oxidative stress factors because as they say “Increased oxidative stress is thought to be one of the underlying factors in the occurrence of AMD.” Factors include smoking and oxLDL.
Mitochondrial Dysfunction
This first article may be the most important on this entire page because of what it implies. It shows that polyunsaturated fatty acids can damage mitochondria. To give you context, in the past, our diets were very low PUFA and so, therefore, was our body fat. But now both are very high in PUFA. This means that we are damaging our mitochondria on a regular basis, both when we eat foods made with vegetable oil and when we burn our body fat.
A criticism of this conclusion might be that it’s based on limited evidence. But I think that if you have gone to school to understand the biochemistry of vegetable oil and the cell physiology of mitochondria and hormone signaling, then you might want to take the time to apply that learning and read the articles for yourself.
Article Title: “Effects of fatty acids on mitochondria: implications for cell death” (PLOS 2016)
- Link (abstract): https://pubmed.ncbi.nlm.nih.gov/12206909/
- Key findings: PUFAs shut down mitochondrial ATP production to the point that would kill cells. Omega-3 are more potent in inducing mitochondria than omega 6. This article supports 1) the finding that uncoupling proteins 2 and 3 that are activated by PUFA and 2) the hypothesis that they do so to protect the cell from ROS, much like a circuit breaker in a house (discussed in detail in The FATBURN Fix) and that this is NOT a good thing.
- Article quotes:
- A first point of interest is that despite their effects as PTP inducers and uncouplers in isolated mitochondria [31] (see also Fig. 2) the saturated myristic, palmitic and stearic acids didn’t cause depolarization of mitochondria within MH1C1 cells, nor did they cause acute cytotoxicity.
- Only arachidic acid (which is ineffective in isolated mitochondria, Fig. 2) caused a small depolarization that wasn’t, however, related to a cytotoxic effect (Fig. 4).
- When monounsaturated fatty acids were compared, it became apparent that only the palmitoleic acid was cytotoxic, cell death being preceded by mitochondrial depolarization.
- Interestingly, the minimal number of unsaturations required to observe both mitochondrial depolarization and the cytotoxic effect increased with the increase of the hydrocarbon chain length.
- Thus, with C18 fatty acids cytotoxicity and mitochondrial depolarization required at least two unsaturations, and increased further for the C18:3 linolenic acid; while with C20 fatty acids at least four unsaturations were needed, cytotoxicity and mitochondrial depolarization being observed only for arachidonic and eicosapentaenoic acids (Fig. 4).
Article Title: “Polyunsaturated Fatty Acids Attenuate Diet-Induced Obesity and Insulin Resistance, Modulating Mitochondrial Respiratory Uncoupling in Rat Skeletal Muscle”
Key Finding: PUFA reduce mitochondrial ATP production. This is bad. But the authors say it’s good!
Author Error: The authors use the term mitochondrial dysfunction in a strange way, even seemingly contradictory. They say that reducing mitochondrial ATP production is a solution to obesity, which is a problem becuase it’s associated with mitochondrial dysfunction and damaging reactive oxygen species. However, reduced ATP production is the very definition of mitochondrial dysfunction, since producing ATP is the job of the mitochondria. This line of thinking is very dangerous. They are suggesting we use our mitochondria to burn off fat without producing ATP, in other words, producing heat. But that heat comes from the release of damaging reactive oxygen species that will destroy mitochondria in the long run, and may lead to cancer. Strange that experts can have such illogical, biologically disastrous ideas.
Key Quote: “mitochondrial dysfunction, increased production of reactive oxygen species (ROS), and impaired mitochondrial biogenesis are considered the key determinants of insulin resistance and obesity. Mitochondrial uncoupling, which reduces the proton gradient across the mitochondrial inner membrane, creates a futile cycle of glucose and fatty acid oxidation without generating ATP [14–17], increasing lipid oxidation and reducing intracellular lipid content . Promoting inefficient metabolism, such as the generation of heat instead of ATP, is a potential treatment for obesity.
Article Title: “Adipose saturation reduces lipotoxic systemic inflammation and explains the obesity paradox”
- Key finding: Omega-6 (LA) depolarize mitochondria (making energy production inefficient/impossible) and promote inflammation
- Finding from Fig 6: C18:2 LA causes more mitochondrial depolarization than C18:1 OA which causes more mitochondrial depolarization than C16:0
“A fish oil diet induces mitochondrial uncoupling and mitochondrial unfolded protein response in epididymal white adipose tissue of mice.”
- Key finding: omega-3 damage mitochondria
- Quote “isocaloric UFD-fed mice have increased expression of mitochondrial uncoupling protein 1 (UCP1) and peroxisomal fatty acid oxidation enzymes suggesting that elevated mitochondrial uncoupling and peroxisomal fatty acid oxidation could contribute to the reduction in fat mass.”
Article Title: “The influence of dietary lipid composition on skeletal muscle mitochondria from mice following 1 month of calorie restriction” [2012]
- Key finding: The more unsaturated a diet, the more oxidative stress is generated in the mitochondria during lipid oxidation as measured by lipid peroxidation (TBARS). By ‘calorie restriction’ they mean the mice were not fed ad lib, which confounds the findings a little but here’s the thing: Usually high fat diets that promote weight gain induce mitochondrial dysfunction and ROS, which is why calorie restriction is thought to be beneficial. This study shows that even in spite of calorie restriction, the PUFA-induce oxidative stress markers in muscle mitochondria are consistent with what you would expect based on the fact that PUFAs seem to damage mitochondria and more higher degree of saturation makes them more damaging. (See article “Effects of fatty acids on mitochondria: implications for cell death”)
- Article Quotes:
- TBARS were measured to assess lipid peroxidation in skeletal muscle mitochondria from the four groups of mice (Figure 3).
- These results indicate that lipid peroxidation is increased in the diet group that consumed the highest amount of n?3 PUFA and had the greatest amount of n?3 PUFA in their mitochondrial membrane.
Muscle Atrophy
A diet containing soybean oil heated for three hours increases adipose tissue weight but decreases body weight in C57BL/6 J mice
https://pubmed.ncbi.nlm.nih.gov/23510583/
Oxidized soy oil consumption (ie from fried foods) promotes increased fat deposition and loss of lean muscle mass.
Article quote
This is the first study to show that a diet containing soybean oil heated for a short time increases fat mass despite a decreased weight gain in C57BL/6 J mice.
Other studies have also reported that animals fed oils heated for 24 h or greater showed reduced weight gain. These observations, while important, have limited significance since fried foods in the typical human diet do not contain the extreme levels of oxidized lipids used in these studies.
The main goal of this study was to investigate the effects of a diet containing soybean oil heated for 3 h on weight gain and fat pad mass in mice.
One explanation for the increased fat pad weight among HSO as compared to USO mice maybe the repartitioning of fat and lean tissue. For example, conjugated linoleic acid, which is very similar in structure to oxidized PUFA (hydroxy linoleic acid), has been shown to change the proportions of lean and fat mass in the absence of weight change [10].
In addition, primary products of lipid oxidation, but not secondary products, have been shown to act as ligands of PPAR?[11]. Activation of PPAR?promotes modest increases in fat mass as seen in mice [12] and humans [13] treated with rosiglitazone, a known PPAR?agonist. We hypothesized that the increase in fat pad mass among mice consuming HSO could be due to an upregulation of PPAR?.
Obesity
High Fat Diets Enriched in Soy Oil promote diabetes and obesity
Linoleic acid causes greater weight gain than saturated fat without hypothalamic inflammation in the male mouse
Dysregulation of Hypothalamic Gene Expression and the Oxytocinergic System by Soybean Oil Diets in Male Mice [Sladek]
Deol P, Fahrmann J, Yang J, et al. Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice. Sci Rep. 2017;7(1):12488.
Deol P, Evans JR, Dhahbi J, et al. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver. PloS One. 2015;10(7):e0132672.
Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism. 1996;45(12):1539–1546.
Omega-3 Fatty Acid Depletion
“The effect of modifying dietary LA and ALA intakes on omega-3 long chain polyunsaturated fatty acid (n-3 LCPUFA) status in human adults: A systematic review and commentary”
Reducing Linoleic acid intake naturally increases omega-3 in a person’s body without the need to supplement omega 3 in the diet or elsewhere. Presumably, this occurs because reducing LA means less oxidation will happening, so less omega-3 destruction occurs and more of what you eat remains in a form your body can use.
Article quote ” The results suggest that it is possible to increase n-3 LCPUFA status by reducing
LA and/or increasing ALA intake in humans, although decreasing LA intake to below 2.5%E may be
required to specifically increase levels of the n-3 LCPUFA docosahexaenoic acid (DHA).”
Oxidized Cholesterol (See Atherosclerosis, PUFA Promote Oxidized LDL and HDL)
https://16376958005805634330.googlegroups.com/attach/2302d4788ee28/reduction%20of%20LA%20t
Pregnancy Complications
Seed oils may promote preterm delivery
Article Title: Fatty acid composition of adipose tissue at term indicates deficiency of arachidonic and docosahexaenoic acid and excessive linoleic acid supply in preterm infants
https://link.springer.com/article/10.1007/s00394-020-02293-2
Schizophrenia
Serum fatty acid patterns in patients with schizophrenia: a targeted metabonomics study
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5538128/#sup1
Monounsaturated fatty acids (MUFAs) and ?-6 polyunsaturated fatty acids (?-6 PUFAs) were significantly increased in SCZs compared with HCs. Desaturation from saturated fatty acids to MUFAs and ?-oxidation were enhanced, as estimated by the ratios of products to precursors. These results suggest that lipolysis and ?-oxidation are upregulated in SCZ, presumably resulting from insufficient brain energy supply.
Stomach Acid Deficit
Linoleic acid excess causes reduced stomach acid
https://www.gastrojournal.org/article/0016-5085(88)90553-7/pdf
Dietary Linoleic Acid, Gastric Acid, and Prostaglandin Secretion
Linoleic acid promotes excessive prostaglandin production which leads to increased gastric mucus and reduced stomach acid, mechanism unclear.
Reduced stomach acid may be associated with increased H Pylori infection? (although H pylori can protect itself from acid with urease) or other increased pathogen colonization, as well as reduced release of pepsin and thus altered digestive function. Need to investigate this more.
Sudden Cardiac Death
This is arguably the most important kind of heart problem to prevent since you have a near-zero chance of surviving it. And once again we find excess dietary PUFA (linoleic acid) plays a role. And again, this is independent of its eicosanoid derivative arachidonic acid, and solely a result of the body’s inability to handle so much PUFA. This matters because it means taking fish oil, even one that is high quality and not already deteriorated into toxins, could very well make the problem worse.
Article title: “Fatty acid composition in total phospholipids of human coronary arteries in sudden cardiac death”
Key findings and commentary:
- “The coronary arteries from cases of sudden cardiac death showed more atherosclerotic lesions than those of controls.”
- This reinforces the fact that the main driver of sudden cardiac death in the population as a whole is coronary artery disease.
- “The percentages of palmitic acid (16:0) and linoleic acid (18:2(n-6)) were significantly higher and the percentage of arachidonic acid (20:4(n-6)) and of all the other major polyunsaturated fatty acids, both n-6 and n-3, was significantly lower in cases of sudden cardiac death than in controls. ”
- The linoleic acid content is higher due to long-term consumption in the diet.
- The palmitic acid concentration is higher most likely due to the body not desaturating it into palmitoleic as a way of trying to adapt to the excess PUFA, which lowers the melting point of membranes. This is a consistent finding across other areas of study.
- “In conclusion, this study showed increased percentages of saturated and reduced percentages of polyunsaturated fatty acids, except linoleic acid, in total phospholipids of human coronary arteries in cases of sudden cardiac death.”
- If you have been wondering how the AHA can justify its position that decreased PUFA is harmful, this is how. It’s not clear from the abstract (which is all most people read) if total PUFA is reduced or just all PUFA other than linoleic acid. But the AHA will take that phrase out of context and run with it.
- “The results suggest an impaired metabolism of linoleic acid, possibly due to a decreased delta-6-desaturase activity in the coronary artery wall in cases of sudden cardiac death.”
- My interpretation is that either chronic excessive LA downregulates delta-6 desaturase, or depletes antioxidants so that the longer and more polyunsaturated derivatives of LA are destroyed faster than they are created.
Sunburn
This may not seem important but it ages our skin and leads to certain kinds of cancers.
The Skin Epilipidome in Stress, Aging, and Inflammation
“ The long-wavelength UVA (320–400 nm) oxidizes lipids in absence of enzymes but also shorter wavelength radiation can non-enzymatically generate oxidized lipids via free radical mechanisms. Cholesterol, phospholipids, free fatty acids, and squalene are targets for non-enzymatic lipid oxidation and yield bioactive products. Enzymatic synthesis of oxidized lipids, most prominently eicosanoids and related oxidized polyunsaturated fatty acids (PUFAs) results from UV activation of phospholipases, lipoxygenases and cyclooxygenases. Most of the work on enzymatic generation of eicosanoids has been done on the response to clinically relevant short wavelength UVB irradiation. This may lead to an underestimation of non-enzymatic effects to solar UV exposure which are mostly elicited by longer wavelength radiation. “
Mechanisms of UV-Induced Inflammation
“Oxygen radical – induced peroxidation of membrane lipids caused by irradiation may contribute to increased phospholipase activity. Oxygen-free radicals also participate in sunburn cell formation and in UV-induced decreases in Langerhans cell numbers. Several enzymatic and non-enzymatic mechanisms are present in skin for reducing these highly reactive oxygen species.”
Conditions Driven by Insulin Resistance
(This concept that multiple diseases share the same root cause as type 2 diabetes has also been called the “Common Soil Hypothesis”)
Cognitive Dysfunction
Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance.
https://www.ncbi.nlm.nih.gov/pubmed/20176121
“a rodent model of type 2 diabetes mellitus produced by a high-fat diet impairs basal cognitive function and attenuates both cognitive and metabolic responses to hippocampal insulin administration”
the high-fat diet was high PUFA https://researchdiets.com/formulas/d12266b as the fat content comes from mostly from corn oil 118g versus butter content of 44.2 g butter
Loss of Self Control (IR induces hypoglycemia symptoms)
Mental Work Requires Physical Energy: Self-Control Is Neither Exception nor Exceptional
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6041938/
Cognitive processes are limited by restraints in brain glucose supply
Brain extracellular glucose does decrease with cognitive activity, and
The magnitude of depletion is correlated with activation of specific brain regions and cognitive processes.
Systemic hypoglycemia does cause cognitive impairment despite blood glucose remaining well above normal brain-extracellular-fluid glucose levels, both in animal studies and in a large number of human studies across multiple cognitive domains (e.g., Cox et al., 1993; Weber et al., 1994; Draelos et al., 1995; Clarke et al., 1999; Strachan et al., 2000; McNay et al., 2010). [McNay et al uses a high pufa diet to induces the IR that causes these effects]
MECHANISMS of TOXICITY: Mitochondrial Dysfunction and Cytotoxicity
“Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice”
What did they do?
Tested 3 diets on mice to see how they affected liver cells. The three diets were all about 40% fat and varied by PUFA content and addition of OXLAMs (a mixture of heated/partly oxidized linoleic acid molecules from used corn oil). The low PUFA contained 4.3% by caloric content Linoleic acid. The high PUFA contained 17% calories from LA. The OxLam diet contained 4.3% calories of the heated corn oil.
What did they find?
The low LA diet was not liver toxic. The high LA diet was. The OXLAM diet was even more liver toxic. The mechanism of toxicity was primarily mitochondrial dysfunction.
Furthermore, the LA diet was much higher in OXLAMS than the low LA diet, showing that OXLAMs develop spontaneously in vivo on a high LA diet. Thus at high concentrations, LA has serious toxicity.
Why it matters?
This study provides evidence that fried food can cause fatty liver as well as mitochondrial dysfunction, thus probably damaging mitochondria other cell types. It also provides evidence that a high PUFA diet (LA specifically) even without thermally stressed oil can do the same, but at a slower rate.
Quote:
Our data showed that a diet rich in LA increases the plasma oxylipin level, reduces hepatic lipogenesis, and increases hepatic FA oxidation, but is not sufficient to induce oxidative damage, lobular inflammation, and NLRP3 inflammasome activation. However, we cannot exclude that long-term feeding with high LA could worsen this outcome, as early signs of mitochondrial dysfunction, such as reduced mitochondrial Complex 1 protein level, lower ATP levels, and increased TXNIP protein levels, were present in mice fed the high LA diet.
Research Errors Making PUFA Look Healthier Than it Is
PUFA in “High Fat” Diets
https://researchdiets.com/formulas/d12266b
Corn oil 118 g, Butter 44.2g per container
PUFA IN LARD
Exploring the Impact of n?6 PUFA-rich Oilseed Production on Commercial Butter Compositions Worldwide
Amy Botta and Sanjoy Ghosh
35% of FA in Lard come from PUFA (much higher than what’s reported in nutrition tables including US government)
Vitamin E in oxidized Seed oils has Pro-Oxidant Activity
Antioxidant compounds can become pro oxidants once ‘used’ if not ‘recharged’
For example, vitamin E. After it’s ‘used’ once to trap a high energy electron, it then acts like a pro oxidant. In the body it gets ‘recharged’ by a combination of vitamin C, glutathione, and several enzymes.
Prooxidant activity of oxidized alpha-tocopherol in vegetable oils
https://pubmed.ncbi.nlm.nih.gov/19895457/
Points of confusion
Antioxidant Supplementation: Mostly Worthless
Antioxidant compounds can become pro oxidants once ‘used’ if not ‘recharged’
For example, vitamin E. After it’s ‘used’ once to trap a high energy electron, it then acts like a pro oxidant. In the body it gets ‘recharged’ by a combination of vitamin C, glutathione, and several enzymes.
Omega-3 supplementation does not help anything
Title: Polyunsaturated fatty acid biosynthesis pathway and genetics. implications for interindividual variability in prothrombotic, inflammatory conditions such as COVID-19 (Nov 2020)
https://www.sciencedirect.com/science/article/pii/S0952327820301411
Key Point: Reducing LA increases omega-3 levels.
- Put another way, only 2 ways are known to increase circulating DHA status in humans: 1) consume (preformed) DHA, or 2) lower dietary LA.
- My comment: It may not be competition. Another possibility is the destruction of substrate n3 due to excessive uncontrolled oxidation of n3 that is reduced by reducing total PUFA.
Title “Reducing the Dietary Omega-6:Omega-3 Utilizing alpha-Linolenic Acid; Not a Sufficient Therapy for Attenuating High-Fat-Diet-Induced Obesity Development Nor Related Detrimental Metabolic and Adipose Tissue Inflammatory Outcomes”
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3986354/
- Article quotes
- Taken together, our findings regarding AT [adipose tissue] phospholipid composition and the outcomes related to macrophage polarization, inflammation, and insulin resistance suggest that changes in phospholipid composition leading to an increase in the EPA:AA and/or DHA:AA may lead to reduced macrophage infiltration, possibly via regulation of CXCL14, but does not necessarily reduce AT inflammation, as assessed by the inflammatory markers measured, nor hinder the development of insulin resistance.
- Dr. Cate’s takeaway: Longer chain HUFA omega-3 supplementation with DHA or EPA is unlikely to perform any better in the goal of resolving inflammation or insulin resistance.
Vitamin E in oils is oxidized and harmful
“Prooxidant activity of oxidized alpha-tocopherol in vegetable oils”
https://pubmed.ncbi.nlm.nih.gov/19895457/
HODE researchers
https://cancer.wisc.edu/research/staff/shanmuganayagam-dhanansayan/
Author of
Modified HPLC method for detection of hydroxyoctadecadienoic acid with greater sensitivity
Dr. Shanmuganayagam researches the role of metabolic syndrome (dysfunctional adipose response) and inflammation (dysfunctional immune response) in aging and the development of aging associated diseases (sp. cancer and cardiovascular disease). Long-term reduction in caloric intake is the most effective means by which the process of aging can be slowed down. Thus, caloric restriction provides a tool for understanding the aging process.
Lipidologist misperceptions around oxidation, antioxidants, PUFAs, and saturated fat:
Lipidology and cardiology do not have a good grasp on the biochemistry of cell membranes and most in this area are working under the incorrect assumption that saturated fat causes oxidative stress. Cell membrane physics, by contrast, is a field where the scientists have a much better understanding of oxidation. The article cited below from AIMS Biphys represents an excellent primer and introduction to how PUFA oxidation disrupts cell membrane physics and function.
PUFA oxidation causes cell membranes to polymerize, becoming rubbery and dysfunctional, with complex consequences that promote accelerated aging of the cell, dysfunction and disease.
Free-radicals and advanced chemistries involved in cell membrane organization influence oxygen diffusion and pathology treatment AIMS Biophys. 2017; 4(2): 240–283.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5707132/
SATURATED FAT
The fact that we see saturated fat present in cell membranes in higher levels in T2DM than in metabolically healthier people has led many to wrongly conclude that saturated fat plays a role in causing T2DM. The reality is that the PUFAs have oxidized and in doing so they have become more saturated. So saturated fat remains. The cause and effect is mixed up, as is so often the case when the assumptions going into an experiment are incorrect.
Because the pathologic nature of diabetes is associated with ROS [13], the resultant ratio change of lower unsaturated to saturated lipids with increased membrane stiffness [37] is a sign of lipid free-radical C=C bond breakdown into aldehydes with loss of unsaturated lipids.
Subsequent formation of lipid breakdown aldehydes can then also result in development of unsaturated aldehyde crosslinker products generated toward free-radical lipid C=C bond chain-growth covalent structural rigidity [1] that could increase membrane stiffness in diabetes [37].
CHOLESTEROL
As with saturated fat, the fact that we see cholesterol present in cell membranes in higher levels in T2DM than in metabolically healthier people has led many to wrongly conclude that high membrane cholesterol plays a causative role in diabetes Example: [Membrane Cholesterol in Skeletal Muscle: A Novel Player in Excitation-Contraction Coupling and Insulin Resistance]Again, because cholesterol is stable to oxidation, after the PUFAs have oxidized, membranes may appear relatively higher in cholesterol than before PUFA oxidizes.
Support for the idea of cholesterol as an antioxidant that reflects the fact that a tissue has been subjected to PUFA-induced oxidative stress: (from https://www.pnas.org/content/115/13/3255)
It has been hypothesized that cholesterol might function as a sacrificial antioxidant since oxidized cholesterols (oxysterols) are often detected in blood (11).
It has also been suggested that cholesterol can lower the damage of phospholipid layers caused by ionizing radiation-initiated oxidation, inferred by indirect means such as the measurement of the fluorescence lifetime of other additives
Human Studies on PUFA need to be longer than they usually are.
Studies that fail to show the harms of high PUFA consumption are usually just a few weeks long, which is very short compared to the human lifespan and too short to be useful. Why? Becuase it takes a long time to deplete the antioxidant capacity of the body.
“The human organism is equipped with very efficient antioxidative defense mechanisms that, among others, include antioxidative enzymes such as SOD, catalase, glutathione peroxidase, and glutathione reductase”
“When the production of ROS is prolonged, the endogenous reserves of antioxidants become insufficient, leading to cell damage. Similarly, the production of ROS below physiological levels induces a decreased proliferative response.”
From “Oxidative Stress and the Aging Brain: From Theory to Prevention” https://www.ncbi.nlm.nih.gov/books/NBK3869/
Essential Functions of PUFA
How PUFA enter/ build brain
Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain.
https://www.ncbi.nlm.nih.gov/pubmed/11478370
Therefore, if the plasma FFA is the primary source of fatty acid for the brain, much of the DHA must be synthesized in the brain from n-3 PUFA precursors. Alternatively, if the brain requires large amounts of preformed DHA, the phospholipids contained in plasma lipoproteins are the most likely source.
Biomarkers
- Title: Adductome-based identification of biomarkers for lipid peroxidation (FT Researchgate)
- Based on the high resolution ESI-MS of the adduct and on the LC-ESI-MS/MS analysis of the synthetic adduct candidates, the major lysine adduct detected in the oxidized LDL was identified as N?-(8-carboxyoctanyl)lysine (COL). Strikingly, a significantly higher amount of COL was detected in the sera from atherosclerosis-prone mice and from patients with hyperlipidemia compared to the controls. These data not only offer structural insights into protein modification by lipid peroxidation products, but also provide a platform for the discovery of biomarkers for human diseases
Estimating Adipose PUFA from NEFA is Accurate
“Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake” [need HML login to access]
NEFA correlates best with adipose FA profile
Cholesterol ester contained 50% LA on average (see fig 7)
“Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity” see table below Garaulet et al.
Estimating Dietary PUFA from Cell Membranes is NOT Accurate
“Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance”
- Aricle Link: https://www.sciencedirect.com/science/article/pii/S0005273612000156#:~:text=PUFA%20levels%20are%20lowest%20in,triglycerides%20(7%E2%80%9355%25)
- Article quotes
- An extensive study of influence of diet fat on membrane composition in rat tissues.
- Extensive changes in diet SFA, MUFA and PUFA had minimal effect on membranes (average slopes 0.01, 0.07, 0.07 respectively), but considerable influence on adipose tissue and plasma triglycerides (average slopes 0.27, 0.53, 0.47 respectively).
- Membrane SFA, MUFA and PUFA homeostatically regulated irrespective of diet variation.
- Adipose tissue and plasma triglyceride composition are highly responsive to diet.
PUFA CONCENTRATION of Cell Membranes
8% WBC from Rats cells cultured on basic medium, add up the numbers on the left hand side of table 1, gets you 8% PUFA.
From “Fatty Acid and Peptide Profiles in Plasma Membrane and Membrane Rafts of PUFA Supplemented RAW264.7 Macrophages”
History of oil use
In Ancient Egypt
http://sites.dlib.nyu.edu/viewer/books/isaw_basp000006/20
SOURCES FOR PUFA DRIVEN DISEASE GRAPH (Top of page)
Seed oil Consumption data sources
Data on Seed Oil Consumption 2000-2020
Consumption is in 1000 metric tons
TOTAL consumption of soy, canola, sunflower and corn
2000: 10,000
2010: 11,203
2019: 17,660
Calculations I used to translate total us consumption in metric tons to individual consumption in ounces per day:
Given baseline per capita consumption estimates from Guyanet in 1999, I used 2000 as a conservative baseline (since consumption actually increased between 1999 and 2000) the 2010 data (11,203) represent increases of 12 percent over the 1999/2000 data, for a figure of 72 ounces per day
The 2019 data (17,660) represents increases of 76% over the 1999/2000 data, for a figure of 105 ounces per day
Prediabetes and diabetes trend data sources
These data show references for the rates of diabetes and prediabetes in the image at the top of this page.
Undiagnosed diabetes was assumed to be 20% of diagnosed diabetes and added to the rates of diagnosed, per slide on the right.
Prediabetes rates in 2020 are approximate 2.5x diabetes rates according to page 8 of the document cited on the right, and this was assumed to be constant over time.
Link to document source for left graph https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
Link to document source for right table https://www.cdc.gov/diabetes/statistics/slides/long_term_trends.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5235953/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5235953/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5235953/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5235953/
Please note: Please do not share personal medical information in a comment on our posts. It will be deleted due to HIPAA regulations.
This Post Has 11 Comments
Note: Please do not share personal information with a medical question in our comment section. Comments containing this content will be deleted due to HIPAA regulations.
















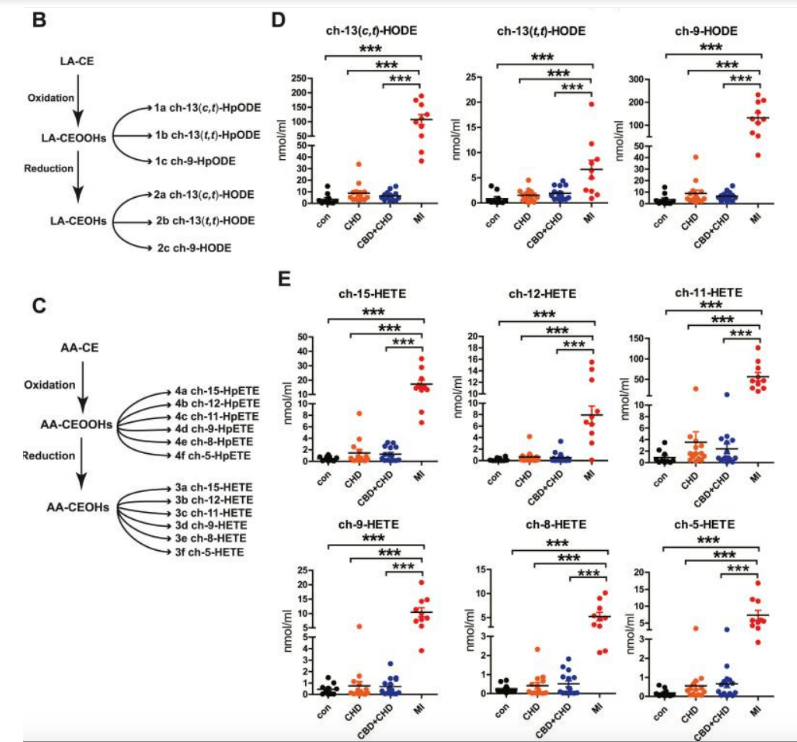
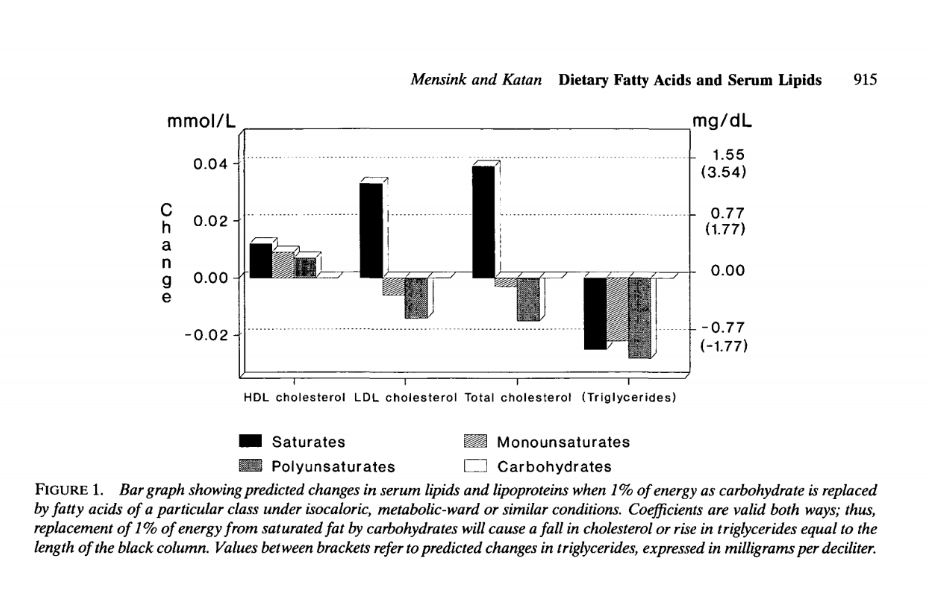
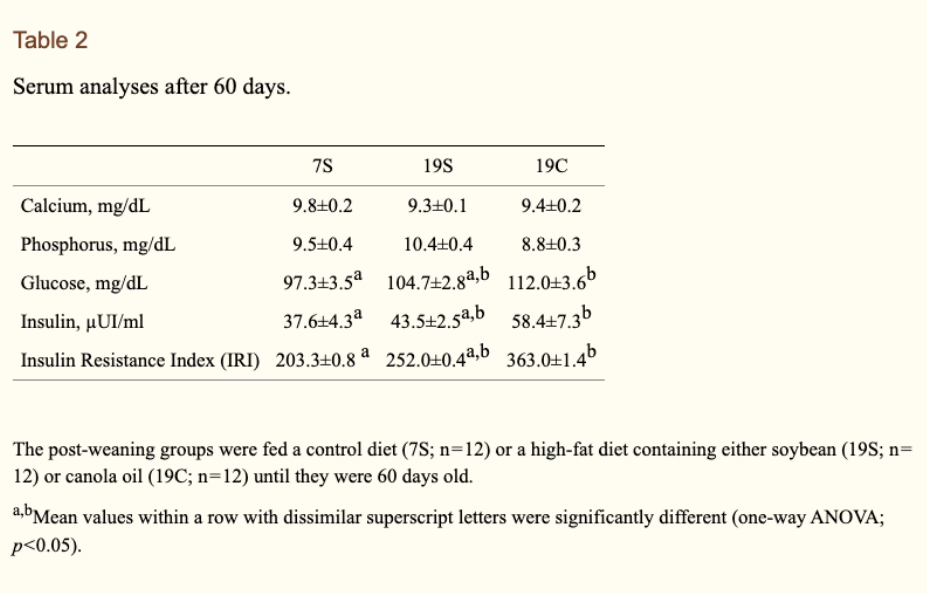
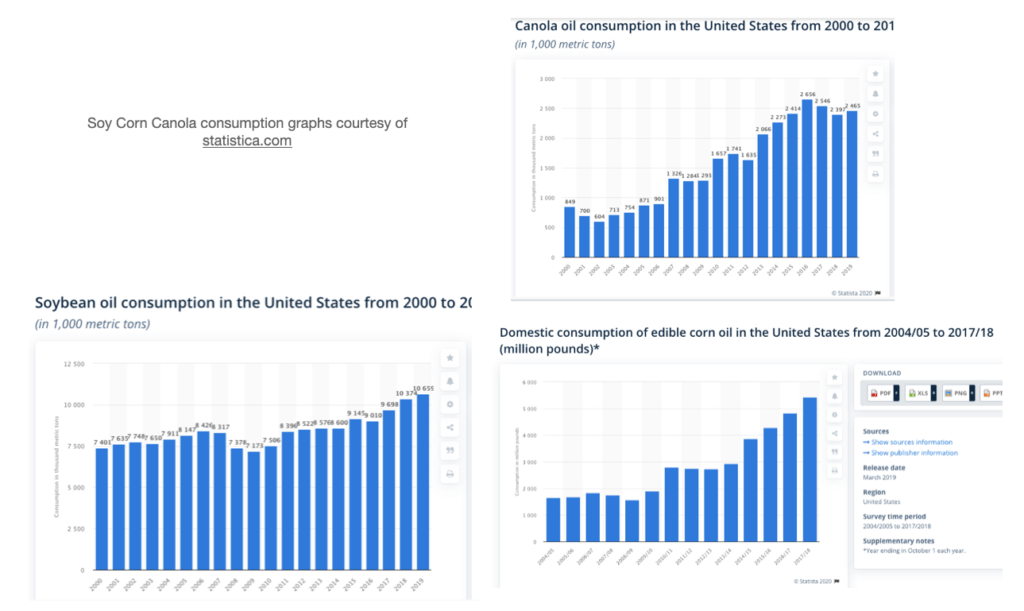
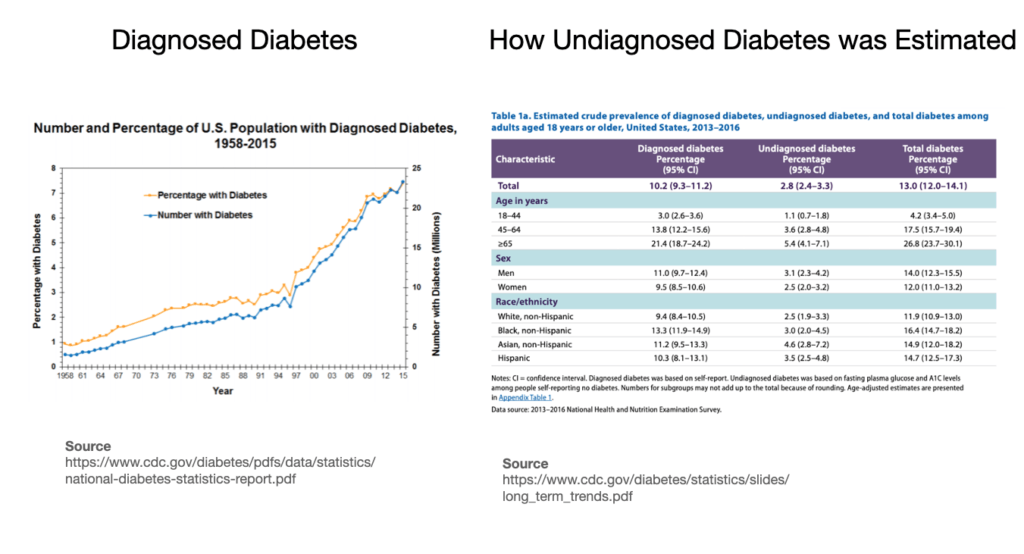



Dr Shanahan is Sunflower Lecithin Oil the same as sunflower seed oil? Is it toxic? Is MCT oil bad? I wanted to try a supplement but discovered it has these 2 items in it.
Hi Sharon I have a few articles on lecithin here: https://drcate.com/?s=lecithin
I don’t have an MCT oil article yet.
Hi Dr. Cate,
I really enjoyed your presentation here: https://youtu.be/1-1isEj2CSc?si=vzaK3EZI6H-S-yWh&t=1157
I might like to repeat the experiment with the mitochondria in the video at 19:17, it is very interesting to me that they cannot burn PUFA fats effectively, could you find the original reference for me?
Thank you for sharing all this eye-opening information.
It’s on my PUFA Project page, and the link takes you right to that study https://drcate.com/pufa-project/#mitochondrial-dysfunction
Do you mean you would literally repeat it? Are you a researcher?
What will be the blood work markers if you are consuming too much PUFA?
Hi Dr Cate – Kristy here from Aotearoa/New Zealand.
I have been following advice in your Fatburn Fix book. I was already on a Ketogenic diet and have cut the hateful 8 from my diet!
I had an abdominal ultrasound today and wonderfully I no longer have any signs of non alcoholic fatty liver, which I’ve had for around 10yrs. I’m stoked!
Unfortunately though, the scan identified I do have fatty pancreas.
Do you think keeping on track with keto and no hateful 8 will heal my fatty pancreas?
Had you lost weight or did the fatty liver resolve without weight loss?
Either way, Great news. Congrats. ABsolutely the fat around the pancreas should resolve, too, especially if you do lose weight.
Hi Dr Cate,
Yes, so far I have lost 14kg since October 2022, and am happy with my new way of eating. I feel a lot better, less hungry and don’t crave damaging foods anymore. I’m so grateful to you for teaching me about the dangers of PUFAs – thank you!
Hi Dr. Cate Shanahan,
I get that I need to eliminate the hateful 8 PUFA seed oils from my diet, but does that also include eliminating all PUFA from diet. For example food labels list the total grams of PUFA from other sources as there are no SEED OILS on the ingredients list.
Thank you
Just the oils. PUFA in whole foods is usually accompanied by antioxidants and so on so is not the same kind of problem.
Test